Abstract
Introduction
Chronic rhinosinusitis (CRS) is strongly associated with significant impairment of quality of life (QoL) in children. The SN-5 questionnaire is an important assessment tool for pediatric CRS. This study aimed to evaluate potential prognostic factors for treatment of pediatric CRS within the Hebrew version of the SN-5 questionnaire.
Methods
A prospective study in pediatric otolaryngology unit. Patients were treated either surgically or pharmacologically. Following informed consent, parents of pediatric CRS patients completed the translated and validated Hebrew version (SN-5H) prior to treatment and after three months. We analyzed the results of both treatment arms according to success (achieving minimal clinically important difference; MCID).
Results
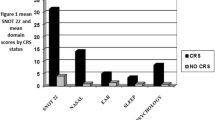
102 children aged 5–12 years and their caregivers participated (74 CRS patients and 28 controls without CRS). SN-5H items scores were significantly higher in CRS patients compared to controls (p < 0.001). Baseline activity scores were higher, while baseline emotional scores were lower in MCID( +) CRS patients, compared to MCID(-) CRS patients (p < 0.05). High emotional stress and low activity scores at baseline were associated with poorer odds to achieve MCID.
Conclusions
The SN-5H questionnaire is invaluable tool for assessing pediatric CRS patients. Psychosocial aspects of CRS significantly affect QoL and should be addressed in the office pre-treatment. The SN-5H can aid in highlighting patients in need for further reassurance and psychosocial support to manage expectations, and to improve QoL.




Similar content being viewed by others
Data Availability
The data that support the findings of this study are available on request from the corresponding author [O.Z].
References
Cunningham, M. J., Chiu, E. J., Landgraf, J. M., & Gliklich, R. E. (2000). The health impact of chronic recurrent rhinosinusitis in children. Archives of otolaryngology--head & neck surgery, 126(11), 1363–1368.
Rudnick, E. F., & Mitchell, R. B. (2006). Improvements in quality of life in children after surgical therapy for Sinonasal disease. Otolaryngology-Head and Neck Surgery, 134(5), 737–740. https://doi.org/10.1016/j.otohns.2005.12.033
Fokkens, W. J., Lund, V. J., Hopkins, C., Hellings, P. W., Kern, R., Reitsma, S., Toppila-Salmi, S., Bernal-Sprekelsen, M., Mullol, J., Alobid, I., Terezinha Anselmo-Lima, W., Bachert, C., Baroody, F., von Buchwald, C., Cervin, A., Cohen, N., Constantinidis, J., De Gabory, L., Desrosiers, M., Diamant, Z., … Zwetsloot, C. P. (2020). European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology, 58(Suppl S29), 1–464. https://doi.org/10.4193/Rhin20.600
Gilani, S., & Shin, J. J. (2017). The burden and visit prevalence of pediatric chronic rhinosinusitis. Otolaryngology-Head and Neck Surgery, 157(6), 1048–1052. https://doi.org/10.1177/0194599817721177
Garratt, A., Schmidt, L., Mackintosh, A., & Fitzpatrick, R. (2002). Quality of life measurement: Bibliographic study of patient assessed health outcome measures. BMJ (Clinical research ed.), 324(7351), 1417. https://doi.org/10.1136/bmj.324.7351.1417
Hopkins, C., Gillett, S., Slack, R., Lund, V. J., & Browne, J. P. (2009). Psychometric validity of the 22-item Sinonasal Outcome Test. Clinical Otolaryngology, 34(5), 447–454.
Stewart, M. G., Witsell, D. L., Smith, T. L., Weaver, E. M., Yueh, B., & Hannley, M. T. (2004). Development and validation of the nasal obstruction symptom evaluation (nose) scale. Otolaryngology-Head and Neck Surgery, 130(2), 157–163. https://doi.org/10.1016/j.otohns.2003.09.016
Kay, D. J., & Rosenfeld, R. M. (2003). Quality of life for children with persistent sinonasal symptoms. Otolaryngology-Head and Neck Surgery, 128(1), 17–26. https://doi.org/10.1067/mhn.2003.41
Gargula, S., Luscan, R., Drummond, D., Denoyelle, F., Couloigner, V., Leboulanger, N., & Simon, F. (2021). French translation and validation of the Sinus and Nasal Quality of Life Survey (SN-5) in children. International Journal of Pediatric Otorhinolaryngology, 145, 110706.
Calvo-Henríquez, C., Valencia-Blanco, B., Boronat-Catalá, B., Maza-Solano, J., Díaz-Anadón, Á., Kahn, S., Moure-Gonzalez, J. D., Faraldo-García, A., & Martinez-Capoccioni, G. (2020). Cross-cultural adaptation of the sinus and nasal quality of life survey (SN-5) to Spanish. International Journal of Pediatric Otorhinolaryngology. https://doi.org/10.1016/j.ijporl.2020.110425
World Health Organization (2015). Process of Translation and Adaption of Instruments. http://www.who.int/substance_abuse/research_tools/translation/en
Bobak, C. A., Barr, P. J., & O’Malley, A. J. (2018). Estimation of an inter-rater intra-class correlation coefficient that overcomes common assumption violations in the assessment of health measurement scales. BMC Medical Research Methodology, 18(1), 93. https://doi.org/10.1186/s12874-018-0550-6
Alobid, I., Benítez, P., Bernal-Sprekelsen, M., Roca, J., Alonso, J., Picado, C., & Mullol, J. (2005). Nasal polyposis and its impact on quality of life: Comparison between the effects of medical and surgical treatments. Allergy, 60(4), 452–458. https://doi.org/10.1111/j.1398-9995.2005.00725.x
Wabnitz, D. A., Nair, S., & Wormald, P. J. (2005). Correlation between preoperative symptom scores, quality-of-life questionnaires, and staging with computed tomography in patients with chronic rhinosinusitis. American Journal of Rhinology, 19(1), 91–96.
Tahamiler, R., Canakcioglu, S., Ogreden, S., & Acioglu, E. (2007). The accuracy of symptom-based definition of chronic rhinosinusitis. Allergy, 62(9), 1029–1032. https://doi.org/10.1111/j.1398-9995.2007.01397.x
Eiser, C., & Morse, R. (2001). A review of measures of quality of life for children with chronic illness. Archives of disease in childhood, 84(3), 205–211. https://doi.org/10.1136/adc.84.3.205
Solans, M., Pane, S., Estrada, M. D., Serra-Sutton, V., Berra, S., Herdman, M., Alonso, J., & Rajmil, L. (2008). Health-related quality of life measurement in children and adolescents: A systematic review of generic and disease-specific instruments. Value in health : The journal of the International Society for Pharmacoeconomics and Outcomes Research, 11(4), 742–764. https://doi.org/10.1111/j.1524-4733.2007.00293.x
Rudnick, E. F., & Mitchell, R. B. (2006). Improvements in quality of life in children after surgical therapy for sinonasal disease. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery, 134(5), 737–740. https://doi.org/10.1016/j.otohns.2005.12.033
Jaeshke, R., Singer, J., & Guyatt, G. (1989). Measurement of health status. Ascertaining the minimal clinically important difference. Controlled Clinical Trials, 10, 407–415.
Mouelhi, Y., Jouve, E., Castelli, C., & Gentile, S. (2020). How is the minimal clinically important difference established in health-related quality of life instruments? Review of anchors and methods. Health and Quality of Life Outcomes, 18(1), 136.
Soler, Z. M., Rosenbloom, J. S., Skarada, D., Gutman, M., Hoy, M. J., & Nguyen, S. A. (2017). Prospective, multicenter evaluation of balloon sinus dilation for treatment of pediatric chronic rhinosinusitis. International Forum of Allergy and Rhinology, 7(3), 221–229.
Ni, J. S., Kompelli, A. R., Nguyen, S. A., Schlosser, R. J., Clemmens, C., & Soler, Z. M. (2018). The sinus and nasal quality of life survey (SN-5) in the Management of Pediatric Chronic Rhinosinusitis: A systematic review and meta-analysis. International Journal of Pediatric Otorhinolaryngology, 111, 162–169.
Norman, G. R., Sloan, J. A., & Wyrwich, K. W. (2003). Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Medical Care, 41, 582–592.
Chmielik, L. P., Mielnik-Niedzielska, G., Kasprzyk, A., Stankiewicz, T., & Niedzielski, A. (2021). Health-related quality of life assessed in children with chronic rhinitis and sinusitis. Children (Basel, Switzerland), 8(12), 1133.
Rolfe, A., Cash-Gibson, L., Car, J., Sheikh, A., & McKinstry, B. (2014). Interventions for improving patients' trust in doctors and groups of doctors. The Cochrane Database of Systematic Reviews, 2014(3), CD004134.
Funding
Nothing to declare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interests to declare.
Ethical approval
This study protocol was reviewed and approved by Rabin Medical Center IRB committee, R20-1922.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zavdy, O., Golan, G., Yaniv, D. et al. Novel prognostic factors for successful treatment of pediatric chronic rhinosinusitis using the sinus and nasal quality of life survey (SN-5H). Qual Life Res 32, 2541–2549 (2023). https://doi.org/10.1007/s11136-023-03421-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-023-03421-1