Abstract
Purpose
This study aimed to determine predictors of health-related quality of life (HRQoL) in Parkinson's disease (PD) and to explore their predictive value before and after controlling overlapping items between HRQoL and clinical variables.
Methods
One hundred and eight PD patients underwent motor, anxiety, depression, apathy, fatigue, and neurocognition assessment. HRQoL was assessed by the Parkinson’s Disease Questionnaire-39 (PDQ-39). In order to determine predictors of HRQoL in PD, stepwise multiple regression analyses were performed in two ways: before and after removing the emotional well-being dimension from PDQ-39 to control the overlap between depression and anxiety, and HRQoL.
Results
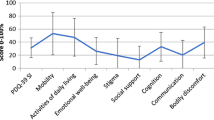
HRQoL total index was predicted by anxiety, fatigue, motor symptoms, and depression, explaining 26.9%, 7.2%, 2.8%, and 1.9% of the variance. However, after removing overlapping items, HRQoL total index was predicted by fatigue (16.5%), anxiety (6.1%), motor symptoms (3.9%), and neurocognition (2.5%), but not depression. Regarding HRQoL dimensions, mobility and activities of daily living were predicted by fatigue (19.7% and 5%) and UPDRS-III (4% and 10.2%); emotional well-being by fatigue (7.9%); social support by anxiety (12.2%) and UPDRS-III (8.6%); communication by neurocognition (5.3%) and UPDRS-III (3.4%); cognition by anxiety (10.6%) and bodily discomfort by anxiety (23%) and fatigue (4.1%).
Conclusion
These findings showed the importance of identifying and controlling overlapping items of HRQoL and clinical measures to perform an accurate interpretation. HRQoL dimensions showed different predictors before and after controlling the overlap. Based on these results fatigue, anxiety, motor symptoms, and neurocognition, but not depression are the main predictors of HRQoL in PD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Plain English summary
Parkinson’s disease is characterized by motor and non-motor symptoms that affect quality of life of Parkinson's disease patients. Health-Related Quality of Life of Parkinson's disease patients has been increasingly studied. Most studies are focused on the assessment of overall Health-Related Quality of Life by Parkinson’s Disease Questionnaire-39. Studies analyze the impact of motor and clinical symptoms, while cognition has not been thoroughly investigated. However, most studies in Parkinson's disease have not considered the possible overlap between clinical and quality of life measures. Additionally, few studies have investigated dimensions of the questionnaire. This study analyzed the influence of motor and non-motor symptoms on Health-Related Quality of Life in Parkinson's disease patients before and after controlling for the overlap between Health-Related Quality of life items and clinical variables. Our results suggest that overall Health-Related Quality of life was influenced by anxiety, fatigue, motor symptoms, and depression, being anxiety the predominant predictor. After controlling overlapping items, results showed that fatigue predominated over anxiety, motor symptoms, and depression. In fact, depression was no longer a predictor and neurocognition emerged as a predictor. Regarding dimensions, fatigue and motor symptoms, influenced mobility and activities of daily living dimensions. Fatigue influenced emotional well-being dimension. Anxiety and motor symptoms influenced social support dimension. Neurocognition and motor symptoms influenced communication dimension. Anxiety influenced cognition dimension, while bodily discomfort dimension was affected by anxiety and fatigue. These findings highlight the importance of identifying and controlling overlapping items of Health-Related Quality of life and clinical measures to perform an accurate interpretation of the results.
Introduction
Assessment of patients’ quality of life has become an important outcome indicator in Parkinson’s disease (PD) [1]. PD patients show an impaired quality of life [2], not only due to the motor symptoms, but also several non-motor symptoms such as sensory abnormalities, sleep disorders, autonomic disturbances, cognitive impairment, and some neuropsychiatric aspects, like anxiety, depression, apathy, and fatigue [3, 4].
Quality of life includes both an individual's perception of themselves in the cultural context and the value systems, and the relationship to their goals, expectations, norms, and concerns [5]. Specifically, health-related quality of life (HRQoL) [2] is defined as the patient's life satisfaction, perception, and self-evaluation of the effects of a disease and their experience with it, being the physical and psychological dimensions, functional capacity, and social interactions the most relevant aspects [2, 6]. The way to assess HRQoL differs among studies and although there are several scales to measure this concept, Parkinson’s Disease Questionnaire-39 (PDQ-39) is the most commonly used instrument for HRQoL assessment in PD [2]. Most of the studies only analyze the overall HRQoL, while PDQ-39 was designed to assess different aspects of the functioning and well-being of people affected by PD [7]. This questionnaire is divided into eight dimensions: mobility, activities of daily living (ADL), emotional well-being, stigma, social support, cognition, communication, and bodily discomfort [7, 8]. Each of these dimensions offers the possibility of analyzing specific aspects that influence the HRQoL of PD patients [7, 8].
On the other hand, cognitive dysfunction is a non-motor symptom of PD that has an impact on the quality of life of PD patients [1]. Cognitive impairment is observed in 40% of people with PD during the course of the disease [9]. The most common deficits in PD involve visuospatial abilities, verbal memory, and frontal executive domains [10]. The risk of progression of cognitive impairment to dementia has a functional impact on the ADL and HRQoL of people with PD [6, 11, 12]. The high association between cognitive impairment and difficulties in ADL persists with disease progression [12] and 83% of PD patients with cognitive impairment develop dementia after 10–20 years [11].
Anxiety, depression, apathy, and fatigue are other characteristic non-motor symptoms that can affect the quality of life of PD patients [13,14,15,16]. Neuropsychiatric problems are present in more than 77% of people with PD [17]. Specifically, meta-analyses showed that the average prevalence rate of anxiety disorders in PD patients was 31% [18], depressive symptoms were found in 22.9% [19], apathy in 40% [16], and fatigue in 50% [14]. Although research generally focuses on depressive symptoms, anxiety is a very common symptom in PD [18] and both have been reported to affect the quality of life of people with PD [15, 20, 21].
A systematic review showed that HRQoL of PD patients has been increasingly explored through diverse studies investigating the predictors of the overall HRQoL in PD [22]. It is essential to identify the most important predictors of HRQoL in PD patients in order to set treatment priorities and reduce the effects of the functional and emotional consequences of PD [22]. However, it should be considered that measures of depression and quality of life are concepts with overlapping items [23,24,25,26], because emotional distress is a construct that is present in most quality of life questionnaires [23, 24], including specific items assessing psychopathology [24, 25], such as depressive [23, 25] and anxiety symptoms. As suggested by Hays and Fayers [23], most of the articles published to date in different pathologies have included measures of depression and HRQoL without considering the empirical and conceptual overlap between the two variables [23]. Therefore, not taking into account the overlap between these measures may result in tautological inferences about the influence of depression on HRQoL, since, if a large proportion of the items overlap, it is logical that quality of life would be significantly associated with measures of psychopathology [23, 24]. Recognition of the overlap between these variables is crucial to interpret associations and avoid incorrect causal inference [23], so it is recommended to control for the presence of psychopathological items in quality of life measures [24].
Conversely, predictors of HRQoL dimensions have not been studied as much as predictors of overall HRQoL [13, 17, 27,28,29,30,31]. Moreover, cognition has not been studied as often as the clinical and motor variables of the disease. Despite some studies having analyzed cognition as a predictor of overall HRQoL [6, 21, 28,29,30, 32,33,34,35], few of them have examined cognition as a predictor of the specific dimensions of HRQoL [28,29,30]. Most of these studies have analyzed cognition with cognitive functioning screening tests, however, further research is needed to analyze the impact of cognition on overall HRQoL and its dimensions using a comprehensive neuropsychological assessment.
Therefore, the main goal of this study was to simultaneously analyze the predictive value of a wide spectrum of variables such as anxiety, depression, apathy, fatigue, cognition, and motor symptoms that impact HRQoL and its dimensions. The second aim of this study was to observe the predictive value of these variables after identifying and controlling the overlap between HRQoL items and clinical measures.
Methods
Participants
One hundred and fifteen PD patients participated in the study between December 2016 and February 2020. Participants were recruited via: (1) the neurologists from the Hospital of Galdakao and the Cruces University Hospital involved in the study, who recruited outpatients regularly attending appointments at the hospital; and (2) ASPARBI and ASOPARA Parkinson's Associations, where informative talks were organized and patients who were members of the associations were recruited. Written informed consent was obtained from all subjects after receiving an explanation of the study. This study was conducted according to the guidelines of Strengthening the Reporting of Observational studies in Epidemiology (STROBE) [36].
The diagnosis of PD based on the diagnostic criteria of the UK PD Society Brain Bank was made by a movement disorder specialist [37]. Other inclusion criteria were as follows: (1) age ≥ 45 and (2) either male or female. The exclusion criteria were as follows: (1) diagnosis of dementia as defined by DSM-IV-TR [38] and the Movement Disorders Society (MDS) specific clinical criteria for PD dementia [39], and (2) diagnosis of other major neurological or psychiatric disorders. All patients received their pharmacological treatment and all evaluations were done in the on medication state. Of the 115 participants, four participants were excluded as a result of other diagnoses (three for atypical Parkinsonism disorders and one for Alzheimer's disease) and three participants dropped out of the study. Finally, the sample was composed of 108 participants.
Demographic, cognitive reserve, and PD-related features assessment
Demographic information, such as age, sex, years of education, and Cognitive Reserve Questionnaire [40] were included. Levodopa Equivalent Daily Dose (LEDD) [41] and years of disease duration were registered. Estimation of the stage and course of the disease was assessed by the Hoehn and Yahr stage Scale (H&Y) [42]. According to MDS level I criteria [43], mild cognitive impairment (MCI) was classified using Montreal cognitive assessment (MoCA) scores, adjusted for age and years of education based on the normative data for the Spanish population [44]. The prevalence in the sample was 56.5%.
Outcome measure
HRQoL was assessed with the Spanish version of PDQ-39 [8]. This questionnaire is divided in eight dimensions: mobility (10 items, 0–40), ADL (6 items, 0–24), emotional well-being (6 items, 0–24), stigma (4 items, 0–16), social support (3 items, 0–12), cognition (4 items, 0–16), communication (3 items, 0–12), and bodily discomfort (3 items, 0–12). The scores for each dimension were calculated by the sum of the raw scores of each item divided by the maximum possible score of each dimension and multiplied by 100 [45]. The PDQ-39 summary index (HRQoL total index) was calculated by the sum of the scores of the 8 dimensions and divided by the number of dimensions [45]. Scores ranged from 0 (no problem) to 100 (maximum level of problems), showing that the higher the score, the lower the HRQoL [45]. An overlap between the items of depression and anxiety measures of the Hospital Anxiety and Depression Scale (HADS) and the emotional well-being dimension items of the PDQ-39 was found (Table 1), so a new HRQoL total index was created without the overlapping items of the emotional well-being dimension.
Potential determining variables
Motor symptoms assessment
Motor symptoms were assessed by the Unified Parkinson's Disease Rating Scale (UPDRS-III) [46].
Anxiety, depression, apathy, and fatigue assessment
Anxiety and depression were measured with the Spanish version of HADS [47], divided into two subscales: seven items of anxiety and seven items of depression. Apathy scores were evaluated with the Spanish version of Lille Apathy Rating Scale (LARS) [48] composed of 33 items. Physical and mental fatigue scores were assessed using the 9-item self-administered Fatigue Severity Scale (FSS) [49].
Neuropsychological assessment
Participants performed an extensive neuropsychological battery in which different cognitive functions were evaluated. Due to the use of multiple measures to assess different cognitive domains, a neurocognition composite score was created including attention and working memory (Digits forward and backward subtest from the Wechsler Adult Intelligence Scale-III [50]), verbal memory (Hopkins Verbal Learning Test-Revised, long-term recall and learning [51]), visual memory (Brief Visual Memory Test-Revised, long-term recall and learning [52]), semantic (Calibrated Ideational Fluency Assessment-Animals category [53]) and phonemic fluency (MoCA language category-fluency subtest [54]), executive functions (UD interference test, Words-Colors and interference [55]), visuoconstructive abilities (Clock Drawing Test, order and copy [56]), cognitive flexibility (Modified Wisconsin Card Sorting Test, categories, and perseverative errors [57]), and processing speed (Salthouse Letter Comparison Test [58] and Trail Making Test-A [59]). All cognitive measures were converted into z scores, and the sign of some measures was adjusted so that higher scores indicated higher cognitive performance. The neurocognition composite score showed a satisfactory internal consistency (α = 0.87).
Statistical analysis
The Kolmogorov–Smirnov test was performed to test normality of data. Missing values were imputed using multiple imputation with the Expectation–Maximization (EM) algorithm. The percentage of missing values was 0.047%.
Spearman’s rho test correlation was used. Firstly, the correlation of HRQoL total index (before and after overlapping items) and its dimensions, with demographic (age and sex), cognitive reserve and LEDD was assessed. These variables were used to adjust the regression models in step 1 as covariates only in the cases that significantly correlated with the HRQoL total index (before and after overlapping items) and the different HRQoL dimensions. The H&Y scale and years of disease duration in PD patients were not included in the model as they were considered global measures of PD severity [60]. Secondly, the relationship of HRQoL total index (before and after overlapping items) and its dimensions, with the potential determining variables (UPDRS-III, anxiety, depression, apathy, fatigue, and neurocognition composite) was assessed. Due to the overlap of items between the emotional well-being dimension and HADS scale, the correlation between the anxiety and depression scores with the emotional well-being dimension was not considered.
Stepwise Multiple Regression analyses were conducted including those variables that correlated significantly in the previous correlation test with the HRQoL total index (before and after overlapping items) and HRQoL dimensions. Regression analyses were adjusted in step 1 (enter method) by covariates showing significant correlations. In step 2 (forward method) motor symptoms, anxiety, depression, apathy, fatigue, and neurocognition scores were included in the models. The statistical analyses were carried out using IBM SPSS statistics v27.
Results
Demographic, cognitive reserve, PD-related features, HRQoL, anxiety, depression, apathy, fatigue, and neurocognition scores of the sample are provided in Table 2.
Correlation analyses of HRQoL and its dimensions
Cognitive reserve was correlated with HRQoL total index (before and after overlapping items), mobility, and emotional well-being dimensions; LEDD variable with HRQoL total index after removing overlapping items, mobility, and communication dimensions; age with mobility dimension and sex with HRQoL total index, emotional well-being, and bodily discomfort dimensions. The correlation coefficients showed weak associations. Consequently, cognitive reserve, LEDD, age and sex variables were included as covariates in the models whose correlation was significant (Table 3).
Regarding the potential determining variables, in general, weak to moderate significant correlations were found between HRQoL total index (before and after overlapping items), several dimensions of HRQoL, and motor symptoms, anxiety, depression, fatigue, and neurocognition scores (Table 4 and Supplementary Material S1).
Predictors of HRQoL and its dimensions
Tolerance values (> 0.1) and variance inflation factor (VIF) values (< 10) of all predictor variables were appropriate, showing that there was no issue with the multicollinearity data.
Stepwise Multiple Regression analyses indicated that the overall model of HRQoL was significant (F (6,101) = 17.77; p < 0.001), showing that HRQoL total index was predicted by scores of anxiety (β = 0.32; p = 0.001), fatigue (β = 0.24; p = 0.003), UPDRS-III (β = 0.17; p = 0.021), and depression (β = 0.18; p = 0.050). When emotional well-being dimension overlapping items were removed, fatigue (β = 0.33; p < 0.001), anxiety (β = 0.25; p = 0.005), UPDRS-III (β = 0.20; p = 0.015), and neurocognition (β = − 0.20; p = 0.038) were predictors of HRQoL total index, and depression was no longer significant (Table 5).
Regarding regressions of HRQoL dimensions, mobility dimension was predicted by fatigue scores (β = 0.41; p < 0.001) and UPDRS-III (β = 0.21; p = 0.013). ADL dimension was predicted by UPDRS-III (β = 0.27; p = 0.004) and fatigue scores (β = 0.23; p = 0.015). Emotional well-being dimension was predicted by fatigue scores (β = 0.28; p = 0.002). Social support dimension was predicted by anxiety scores (β = 0.39; p < 0.001) and UPDRS-III (β = − 0.30; p = 0.001). Cognition dimension was predicted by anxiety scores (β = 0.33; p = 0.001). Communication dimension was predicted by neurocognition (β = − 0.23; p = 0.013) and UPDRS-III (β = 0.19; p = 0.040). Bodily discomfort dimension was predicted by anxiety (β = 0.41; p < 0.001) and fatigue scores (β = 0.22; p = 0.012) (Table 5). Stigma dimension did not show significant correlations, so their regression analyses were not performed (Table 4).
Discussion
The aim of this study was to investigate the predictors of HRQoL and its dimensions in PD patients. Specifically, this study is a multidimensional predictive model that analyzes the predictor value of motor and non-motor symptoms, including scores of anxiety, depression and fatigue and neurocognitive functions in HRQoL and its dimensions in PD. Moreover, this study analyzed the predictive value of these variables after considering the overlapping items between HRQoL and clinical measures.
This study has identified several characteristics associated with a worse HRQoL in PD patients. Higher scores for anxiety, fatigue, motor symptoms, and depression were the factors that predicted a worse HRQoL, with anxiety being the main predictor. The variance in the predictive value of depression scores was lower than in the other studies, with anxiety, fatigue, and motor symptoms predominating over depression scores. Most studies showed that depression was the main predictor of HRQoL [22], and it may be due to the absence of anxiety and depression variables joined in the same regression model [21]. Additionally, the high association between depressive symptoms and quality of life may be due to the overlap between the emotional items of the quality of life measures and depressive symptomatology [25], so these results should be interpreted with caution. Therefore, in our study, an additional analysis was performed in which the emotional well-being dimension items of the HRQoL total index were removed to consider this overlap. Interestingly, our results showed that after taking into account the overlap, fatigue predominated over anxiety and motor symptoms; but depression was no longer a predictor of HRQoL total index. Most studies have not considered the overlap between clinical and HRQoL measures in their analyses and it may be the main reason why depression has been considered as the main predictor of HRQoL. Future studies should contemplate the overlap between clinical and HRQoL measures to perform more accurate interpretations. Controlling the overlapping, fatigue was the main predictor of HRQoL total index. Fatigue is recognized as one of the most disabling symptoms of PD [61], present in 50% of PD patients [14], however, it has not been such a studied predictor as anxiety and depression. In four of the five studies reviewed in a systematic review, fatigue was a strong predictor of HRQoL [22], demonstrating that it emerges in the early stages of PD and persists throughout the course of the disease, negatively impacting their HRQoL [14]. Additionally, in our study, neurocognition emerged as a predictor of HRQoL total index when controlling the overlap. The majority of the studies analyzed cognition with cognitive functioning screening tests [21, 28, 29, 32,33,34,35]. In contrast, in our study, participants performed an extensive neuropsychological battery comprised of a wide variety of cognitive tests, revealing that neurocognition composite score predicted HRQoL. To the authors´ knowledge, only four studies found cognition as a predictor of HRQoL total index [6, 29, 30, 33]. Two of these studies analyzed cognition with cognitive functioning screening tests [29, 33], while the two other studies assessed cognition by specific cognitive measures, showing that working memory [30], verbal fluency [30], visual attention/memory [6], visuospatial [6], and executive functioning [6] were predictors of HRQoL. As far as the authors are aware, this is the first study investigating predictors of HRQoL in PD patients in which the possible overlap between HRQoL and clinical outcomes was analyzed. Furthermore, it should be emphasized that the methodology used and the results obtained in this study could be extrapolated to other pathologies.
Regarding the specific HRQoL dimensions, mobility and ADL dimensions were predicted by fatigue and UPDRS-III. Similar to our results, other studies found that reduced activity and motor symptoms were associated with mobility [27] and ADL dimension [27, 28, 31], while physical fatigue was also a predictor of mobility dimension [62]. Consistent with previous studies [27], our findings showed that fatigue was also a predictor of emotional well-being dimension. Other research suggest that longer disease duration [27], UPDRS total score [27], working memory and verbal fluency [30] were also a predictors of this dimension. Finally, fatigue along with anxiety scores, were predictors of the bodily discomfort dimension. Other studies found that fatigue, UPDRS and female sex [27], anxiety [13, 17, 28], depression and hallucinations [17], and motor fluctuations [28] were predictors of this dimension. However, although in our study depression scores were included in the bodily discomfort model, anxiety and fatigue scores showed a higher association with this dimension, predominating over depression scores. In line with other studies [17, 28], social support dimension was predicted by anxiety scores and UPDRS-III. Additionally, anxiety scores also predicted the cognition dimension. PDQ-39 items of the cognition dimension assess concentration problems, the sensation of having a bad memory and hallucinations or nightmares, which generate feelings of anxiety or nervousness in PD patients. Even though we expected to find neurocognition as a predictor of this dimension, it did not happen. This may be due to the fact that the sensations of concentration problems and memory impairment approach a similar interpretation of cognition; while hallucinations or nightmares are more related to anxiety. In fact, one study suggests that the dimension of cognition in PDQ-39 has a stronger relationship with mood states rather than with neurocognitive domains [63]. Interestingly, neurocognition was found as a predictor of communication dimension, in addition to motor symptoms. The range of communication impairment is very variable in PD (from a lack of problems to inaudible and unintelligible speech) [64]. Indeed, acoustic speech deficits, use of action verbs and pausing have been shown to be more associated with motor impairment and linguistic deficits with cognitive impairment [64].
Concerning stigma, no significant associations were found, so they could not be included in the model. In contrast, other studies showed that anxiety [13, 28], reduced activity and motivation [27], and depression [31] predicted the stigma dimension in PD. Low scores on the stigma dimension may explain the lack of association with the rest of the variables. Furthermore, as Tu and colleagues noted [31], understanding stigma requires a broader consideration from a more social context, and not only focusing on motor and non-motor symptoms. Regarding the rest of the variables, our results did not show a significant association between apathy scores and HRQoL or any of its dimensions. However, other studies revealed an association of apathy with poorer HRQoL [17, 60] and with an increased self-rating of cognition and communication difficulties [17].
Recognition and treatment of motor and non-motor symptoms in the early stages of the disease may improve the HRQoL of PD patients. Considering that PD is a physically and psychologically debilitating disorder, health care professionals should adopt a holistic approach to PD rehabilitation [65]. Treatment objectives vary among individuals, highlighting the need for personalized intervention; so, a variety of non-pharmacological therapies should also be taken into account [66], such as, mindfulness yoga [65], psychological interventions [67], spiritual resilience [68], physical therapy [69], cognitive training [70], and speech therapies [71]. These therapies could be included in the clinical manage of PD patients.
It should be noted that the results of our study were based on cross-sectional data, limiting the ability to observe the impact of motor and non-motor symptoms on HRQoL in the disease course. For future research, longitudinal analyses would be needed to determine if the predictive symptom intervention could affect HRQoL. In fact, a recent longitudinal study examining predictors of HRQoL impairment in PD patients showed that, after a 2-year follow-up, age, sex, mood, and non-motor impairment were associated with clinically significant HRQoL impairment in PD patients [72]. On the other hand, the HRQoL evaluated using PDQ-39 does not allow to contrast the results with other pathologies, since the PDQ-39 is a specific PD scale. In this study, PD patients did not have a long disease duration and were mildly to moderately affected, without major impairments in their HRQoL, so it could be interesting to analyze the predictors of HRQoL and its dimensions in more advanced stages of the disease. Additionally, the low sample size limits generalization, so it would be interesting to conduct future studies with larger sample sizes. In fact, our study was carried out in a Spanish population and there are studies using PDQ-8, which is an abbreviated scale of PDQ-39, suggesting that geographic location has an effect on the non-motor symptoms affecting HRQoL [73]. Specifically, mood and sleep were the major predictors in European patients, whereas, non-motor symptoms were not significant predictors of HRQoL in Indian and Japanese patients [73] and in Chinese population anxiety, depression, motor symptoms, and marital status were the main predictors of HRQoL [74].
In conclusion, our results showed that anxiety, fatigue, motor symptoms, and depression were the main predictors of HRQoL total index in PD patients, whereas, after removing emotional well-being overlapping items, fatigue, anxiety, motor symptoms, and neurocognition were the predictors of HRQoL total index. These findings indicate the importance of identifying and controlling the overlap of items between HRQoL measures and clinical variables in order to perform an accurate interpretation of the results. Our results and methodology would be extrapolated to other pathologies. Additionally, results showed the impact of fatigue, anxiety, motor symptoms, and neurocognition scores in HRQoL dimensions in PD patients. Consequently, results suggest the importance of the appropriate assessment of HRQoL and its specific dimensions, because predictors are different for each dimension of HRQoL. Recognition and management of motor and non-motor symptoms in the early stages of the disease are essential, as these features have a greater impact on the HRQoL of people with PD. Therefore, intervention on fatigue, anxiety, motor symptoms, and cognitive processes may be a crucial target to improving HRQoL in PD patients.
Data availability
The data that support the findings of this study are available on reasonable request from the corresponding author.
References
Tang, Y., Liang, X., Han, L., Peng, F., Shen, B., Yu, H., Shen, Y., Shen, C., Yu, J., & Wang, J. (2020). Cognitive function and quality of life in Parkinson’s disease: A cross-sectional study. Journal of Parkinson’s Disease, 10(3), 1209–1216. https://doi.org/10.3233/JPD-202097
Martínez-Martín, P., Jeukens-Visser, M., Lyons, K. E., Rodriguez-Blazquez, C., Selai, C., Siderowf, A., Welsh, M., Poewe, W., Rascol, O., Sampaio, C., Stebbins, G. T., Goetz, C. G., & Schrag, A. (2011). Health-related quality-of-life scales in Parkinson’s disease: Critique and recommendations. Movement Disorders, 26(13), 2371–2380. https://doi.org/10.1002/mds.23834
Poewe, W., Seppi, K., Tanner, C. M., Halliday, G. M., Brundin, P., Volkmann, J., Schrag, A.-E., & Lang, A. E. (2017). Parkinson disease. Nature Reviews Disease Primers, 3, 1–21. https://doi.org/10.1038/nrdp.2017.13
Schapira, A. H. V., Chaudhuri, K. R., & Jenner, P. (2017). Non-motor features of Parkinson disease. Nature Reviews Neuroscience, 18(7), 435–450. https://doi.org/10.1038/nrn.2017.62
Group, & W. (1993). Study protocol for the World Health Organization project to develop a Quality of Life assessment instrument (WHOQOL). Quality of Life Research, 2, 153–159. https://doi.org/10.1007/BF00435734
Klepac, N., Trkulja, V., Relja, M., & Babić, T. (2008). Is quality of life in non-demented Parkinson’s disease patients related to cognitive performance? A clinic-based cross-sectional study. European Journal of Neurology, 15(2), 128–133. https://doi.org/10.1111/j.1468-1331.2007.02011.x
Jenkinson, C., Fitzpatrick, R., Peto, V., Greenhall, R., & Hyman, N. (1997). The Parkinson’s disease questionnaire (PDQ-39): Development and validation of a Parkinson’s disease summary index score. Age and Ageing, 26(5), 353–357. https://doi.org/10.1093/ageing/26.5.353
Martínez-Martín, P., & Frades, B. (1998). Quality of life in Parkinson’s disease: Validation study of the PDQ-39 Spanish version. The Group Centro for Study of Movement Disorders. Journal of neurology, 245, S34–S38. https://doi.org/10.1007/pl00007737
Baiano, C., Barone, P., Trojano, L., & Santangelo, G. (2020). Prevalence and clinical aspects of mild cognitive impairment in Parkinson’s disease: A meta-analysis. Movement Disorders, 35(1), 45–54. https://doi.org/10.1002/mds.27902
Curtis, A. F., Masellis, M., Camicioli, R., Davidson, H., & Tierney, M. C. (2019). Cognitive profile of non-demented Parkinson’s disease: Meta-analysis of domain and sex-specific deficits. Parkinsonism and Related Disorders, 60, 32–42. https://doi.org/10.1016/j.parkreldis.2018.10.014
Hely, M. A., Reid, W. G. J., Adena, M. A., Halliday, G. M., & Morris, J. G. L. (2008). The Sydney Multicenter Study of Parkinson’s disease: The inevitability of dementia at 20 years. Movement Disorders, 23(6), 837–844. https://doi.org/10.1002/mds.21956
Rosenthal, E., Brennan, L., Xie, S., Hurtig, H., Milber, J., Weintraub, D., Karlawish, J., & Siderowf, A. (2010). Association between cognition and function in patients with Parkinson disease with and without dementia. Movement Disorders, 25(9), 1170–1176. https://doi.org/10.1002/mds.23073
Jones, J. D., Butterfield, L. C., Song, W., Lafo, J., Mangal, P., Okun, M. S., & Bowers, D. (2015). Anxiety and depression are better correlates of Parkinson’s disease quality of life than apathy. Journal of Neuropsychiatry and Clinical Neurosciences, 27(3), 213–218. https://doi.org/10.1176/appi.neuropsych.13120380
Siciliano, M., Trojano, L., Santangelo, G., De Micco, R., Tedeschi, G., & Tessitore, A. (2018). Fatigue in Parkinson’s disease: A systematic review and meta-analysis. Movement Disorders, 33(11), 1712–1723. https://doi.org/10.1002/mds.27461
Schrag, A. (2006). Quality of life and depression in Parkinson’s disease. Journal of the Neurological Sciences, 248(1–2), 151–157. https://doi.org/10.1016/j.jns.2006.05.030
den Brok, M. G. H. E., van Dalen, J. W., van Gool, W. A., Moll van Charante, E. P., de Bie, R. M. A., & Richard, E. (2015). Apathy in Parkinson’s disease: A systematic review and meta-analysis. Movement Disorders, 30(6), 759–769. https://doi.org/10.1002/mds.26208
McKinlay, A., Grace, R. C., Dalrymple-Alford, J. C., Anderson, T., Fink, J., & Roger, D. (2008). A profile of neuropsychiatric problems and their relationship to quality of life for Parkinson’s disease patients without dementia. Parkinsonism and Related Disorders, 14(1), 37–42. https://doi.org/10.1016/j.parkreldis.2007.05.009
Broen, M. P. G., Narayen, N. E., Kuijf, M. L., Dissanayaka, N. N. W., & Leentjens, A. G. (2016). Prevalence of anxiety in Parkinson’s disease: A systematic review and meta-analysis. Movement Disorders, 31(8), 1125–1133. https://doi.org/10.1002/mds.26643
Goodarzi, Z., Mrklas, K. J., Roberts, D. J., Jette, N., Pringsheim, T., & Holroyd-Leduc, J. (2016). Detecting depression in Parkinson disease. Neurology, 87(4), 426–437. https://doi.org/10.1212/wnl.0000000000002898
Carod-Artal, F. J., Ziomkowski, S., Mourão Mesquita, H., & Martínez-Martin, P. (2008). Anxiety and depression: Main determinants of health-related quality of life in Brazilian patients with Parkinson’s disease. Parkinsonism and Related Disorders, 14(2), 102–108. https://doi.org/10.1016/j.parkreldis.2007.06.011
Hanna, K. K., & Cronin-Golomb, A. (2012). Impact of anxiety on quality of life in Parkinson’s disease. Parkinson’s Disease. https://doi.org/10.1155/2012/640707
Soh, S. E., Morris, M. E., & McGinley, J. L. (2011). Determinants of health-related quality of life in Parkinson’s disease: A systematic review. Parkinsonism and Related Disorders, 17(1), 1–9. https://doi.org/10.1016/j.parkreldis.2010.08.012
Hays, R. D., & Fayers, P. M. (2021). Overlap of depressive symptoms with health-related quality-of-life measures. PharmacoEconomics, 39(6), 627–630. https://doi.org/10.1007/s40273-020-00972-w
Katschnig, H. (2006). Quality of life in mental disorders. World Psychiatry, 5(3), 139–145. https://doi.org/10.4088/jcp.v60n0712b
Aigner, M., Förster-Streffleur, S., Prause, W., Freidl, M., Weiss, M., & Bach, M. (2006). What does the WHOQOL-Bref measure? Measurement overlap between quality of life and depressive symptomatology in chronic somatoform pain disorder. Social Psychiatry and Psychiatric Epidemiology, 41(1), 81–86. https://doi.org/10.1007/s00127-005-0997-8
Berlim, M. T., & Fleck, M. P. (2007). Quality of life and major depression. In M. S. Ritsne & A. G. Awad (Eds.), Quality of life impairment in schizophrenia, mood and anxiety disorders (pp. 241–252). Springer.
Havlikova, E. (2008). Impact of fatigue on quality of life in patients with Parkinson’s disease. European Journal of Neurology, 15(5), 475–80. https://doi.org/10.1111/j.1468-1331.2008.02103.x
Simpson, J., Lekwuwa, G., & Crawford, T. (2014). Predictors of quality of life in people with Parkinson’s disease: Evidence for both domain specific and general relationships. Disability and Rehabilitation, 36(23), 1964–1970. https://doi.org/10.3109/09638288.2014.883442
Ophey, A., Eggers, C., Dano, R., Timmermann, L., & Kalbe, E. (2018). Health-related quality of life subdomains in patients with Parkinson’s disease: The role of gender. Parkinson’s Disease. https://doi.org/10.1155/2018/6532320
Stegemöller, E. L., Nocera, J., Malaty, I., Shelley, M., Okun, M. S., & Hass, C. J. (2014). Timed up and go, cognitive, and quality-of-life correlates in Parkinson’s disease. Archives of Physical Medicine and Rehabilitation, 95(4), 649–655. https://doi.org/10.1016/j.apmr.2013.10.031
Tu, X. J., Hwang, W. J., Ma, H. I., Chang, L. H., & Hsu, S. P. (2017). Determinants of generic and specific health-related quality of life in patients with Parkinson’s disease. PLoS ONE, 12(6), 1–12. https://doi.org/10.1371/journal.pone.0178896
Kadastik-Eerme, L., Rosenthal, M., Paju, T., Muldmaa, M., & Taba, P. (2015). Health-related quality of life in Parkinson’s disease: A cross-sectional study focusing on non-motor symptoms. Health and Quality of Life Outcomes, 13(1), 1–8. https://doi.org/10.1186/s12955-015-0281-x
Schrag, A., Jahanshahi, M., & Quinn, N. (2000). What contributes to quality of life in patients with Parkinson’s disease? Journal of Neurology Neurosurgery and Psychiatry, 69(3), 308–312. https://doi.org/10.1136/jnnp.69.3.308
Sławek, J., Derejko, M., & Lass, P. (2005). Factors affecting the quality of life of patients with idiopathic Parkinson’s disease-a cross-sectional study in an outpatient clinic attendees. Parkinsonism and Related Disorders, 11(7), 465–468. https://doi.org/10.1016/j.parkreldis.2005.04.006
Cubo, E., Rojo, A., Ramos, S., Quintana, S., Gonzalez, M., Kompoliti, K., & Aguilar, M. (2002). The importance of educational and psychological factors in Parkinson’s disease quality of life. European Journal of Neurology, 9(6), 589–593. https://doi.org/10.1046/j.1468-1331.2002.00484.x
Von Elm, E., Altman, D. G., Egger, M., Pocock, S. J., Gøtzsche, P. C., & Vandenbroucke, J. P. (2007). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. PloS Medicine, 4(10), 296.
Hughes, A. J., Daniel, S. E., Kilford, L., & Lees, A. J. (1992). Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. Journal of Neurology Neurosurgery and Psychiatry, 55(3), 181–184. https://doi.org/10.1136/jnnp.55.3.181
American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Association.
Emre, M., Aarsland, D., Brown, R., Burn, D. J., Duyckaerts, C., Mizuno, Y., Broe, G. A., Cummings, J., Dickson, D. W., Gauthier, S., Goldman, J., Goetz, C., Korczyn, A., Lees, A., Levy, R., Litvan, I., McKeith, I., Olanow, W., Poewe, W., … Dubois, B. (2007). Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Movement Disorders, 22(12), 1689–1707. https://doi.org/10.1002/mds.21507
Rami, L., Valls-Pedret, C., Bartrés-Faz, D., Caprile, C., Solé-Padullés, C., Castellví, M., Cladera, J. O., Capdevila, B. B., & Molinuevo, J. L. (2011). Cuestionario de reserva cognitiva Valores obtenidos en población anciana sana y con enfermedad de Alzheimer. Revista de Neurologia, 52(4), 195–201. https://doi.org/10.33588/rn.5204.2010478
Tomlinson, C. L., Stowe, R., Patel, S., Rick, C., Gray, R., & Clarke, C. E. (2010). Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Movement Disorders, 25(15), 2649–2653. https://doi.org/10.1002/mds.23429
Hoehn, M. M., & Yahr, M. D. (1967). Parkinsonism: Onset, progression, and mortality. Neurology, 17(5), 427–442. https://doi.org/10.1212/WNL.17.5.427
Litvan, I., Goldman, J. G., Tröster, A. I., Schmand, B. A., Weintraub, D., Petersen, R. C., Mollenhauer, B., Adler, C. H., Marder, K., Williams-Gray, C. H., Aarsland, D., Kulisevsky, J., Rodriguez-Oroz, M. C., Burn, D. J., Barker, R. A., & Emre, M. (2012). Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement disorder society task force guidelines. Movement Disorders, 27(3), 349–356. https://doi.org/10.1002/mds.24893
Ojeda, N., del Pino, R., Ibarretxe-Bilbao, N., Schretlen, D. J., & Peña, J. (2016). Montreal cognitive assessment test: Normalization and standardization for Spanish population. Revista de Neurologia, 63(11), 488–496. https://doi.org/10.33588/rn.6311.2016241
Peto, V., Jenkinson, C., Fitzpatrick, R., & Greenhall, R. (1995). The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Quality of Life Research, 4(3), 241–248. https://doi.org/10.1007/BF02260863
Martínez-Martín, P., Gil-Nagel, A., Gracia, L. M., Gómez, J. B., Martínez-Sarriés, J., & Bermejo, F. (1994). Unified Parkinson’s disease rating scale characteristics and structure. Movement Disorders, 9(1), 76–83. https://doi.org/10.1002/mds.870090112
Quintana, J. M., Padierna, A., Esteban, C., Arostegui, I., Bilbao, A., & Ruiz, I. (2003). Evaluation of the psychometric characteristics of the Spanish version of the Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica, 107(3), 216–221. https://doi.org/10.1034/j.1600-0447.2003.00062.x
García-Ramos, R., Villanueva, C., Catalán, M. J., Reig-Ferrer, A., & Matías-Guíu, J. (2014). Validation of a Spanish version of the lille apathy rating scale for Parkinson’s disease. The Scientific World Journal, 2014, 1–7. https://doi.org/10.1155/2014/849834
Krupp, L. B., LaRocca, N. G., Muir-Nash, N. G., & Steinberg, A. D. (1988). The fatigue severity scale. Journal of Neurology, 46(10), 1121–1123. https://doi.org/10.1007/BF00314361
Wechsler, D. (2001). WAIS-III. Escala de Inteligencia de Wechsler para Adultos. TEA ediciones.
Brandt, J., & Benedict, R. (2001). Hopkins verbal learning test-revised: Professional manual. Psychological Assessment Resources, Inc.
Benedict, R. (1997). Brief visuoespatial memory test-revised: Professional manual. Psychological Assessment Resources, Inc.
Schretlen, D. J., & Vannorsdall, T. D. (2010). Calibrated ideational fluency assesment (CIFA). Psychological Assesment Resources, Inc.
Nasreddine, Z. S., Phillips, N. A., Bedirian, V., Charbonneau, S., Whitehead, V., Collin, I., Cummings, J. L., & Chertkow, H. (2005). The Montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of American Geriatrics Society, 53(4), 695–699. https://doi.org/10.1029/WR017i002p00410
Sáez-Atxukarro, O., del Pino Sáez, R., Peña Lasa, J., Schretlen, D. J., Ibarretxe-Bilbao, N., & Ojeda, N. (2021). UD interference test: Creation and validation of a new instrument of resistance to interference. Normalization and standardization for Spanish population. Neurologia, 21, 1. https://doi.org/10.1016/j.nrl.2021.01.014
Cacho, J., García-García, R., Arcaya, J., Vicente, J. L., & Lantada, N. (1999). Una propuesta de aplicación y puntuación del test del reloj en la enfermedad de Alzheimer. Revista de Neurologia, 28(7), 648–655. https://doi.org/10.33588/rn.2912.99445
Ojeda, N., Peña, J., Ibarretxe-Bilbao, N., & Del Pino, R. (2010). Test de Clasificación de Tarjetas de Wisconsin- Modificado (M-WCST). TEA ediciones.
Salthouse, T. A., & Babcock, R. L. (1991). Decomposing adult age differences in working memory. Developmental Psychology, 27(5), 763–776. https://doi.org/10.1037/0012-1649.27.5.763
Reitan, R. M., & Wolfson, D. (1985). The halstead-reitan neuropsychological test battery: Theory and clinical interpretation (Vol.4). Reitan neuropsychology.
Kuhlman, G. D., Flanigan, J. L., Sperling, S. A., & Barrett, M. J. (2019). Predictors of health-related quality of life in Parkinson’s disease. Parkinsonism and Related Disorders, 65, 86–90. https://doi.org/10.1016/j.parkreldis.2019.05.009
Friedman, J. H., Alves, G., Hagell, P., Marinus, J., Marsh, L., Martinez-Martin, P., Goetz, C. G., Poewe, W., Rascol, O., Sampaio, C., Stebbins, G., & Schrag, A. (2010). Fatigue rating scales critique and recommendations by the Movement Disorders Society Task Force on rating scales for Parkinson’s disease. Movement Disorders, 25(7), 805–822. https://doi.org/10.1002/mds.22989
Elbers, R. G., Van Wegen, E. E. H., Verhoef, J., & Kwakkel, G. (2014). Impact of fatigue on health-related quality of life in patients with Parkinson’s disease: A prospective study. Clinical Rehabilitation, 28(3), 300–311. https://doi.org/10.1177/0269215513503355
Jones, J. D., Hass, C., Mangal, P., Lafo, J., Okun, M. S., & Bowers, D. (2014). The cognition and emotional well-being indices of the Parkinson’s disease questionnaire-39: What do they really measure? Parkinsonism and Related Disorders, 20(11), 1236–1241. https://doi.org/10.1016/j.parkreldis.2014.09.014
Smith, K. M., & Caplan, D. N. (2018). Communication impairment in Parkinson’s disease: Impact of motor and cognitive symptoms on speech and language. Brain and Language, 185, 38–46. https://doi.org/10.1016/j.bandl.2018.08.002
Kwok, J. Y. Y., Kwan, J. C. Y., Auyeung, M., Mok, V. C. T., Lau, C. K. Y., Choi, K. C., & Chan, H. Y. L. (2019). Effects of mindfulness yoga vs stretching and resistance training exercises on anxiety and depression for people with Parkinson Disease: A randomized clinical trial. JAMA Neurology, 76(7), 755–763. https://doi.org/10.1001/jamaneurol.2019.0534
Bloem, B. R., Okun, M. S., & Klein, C. (2021). Parkinson’s disease. The Lancet, 397(10291), 2284–2303. https://doi.org/10.1016/S0140-6736(21)00218-X
Yang, S., Sajatovic, M., & Walter, B. L. (2012). Psychosocial interventions for depression and anxiety in Parkinson’s disease. Journal of Geriatric Psychiatry and Neurology, 25(2), 113–121. https://doi.org/10.1177/0891988712445096
Kwok, J. Y. Y., Choi, E. P. H., Chau, P. H., Wong, J. Y. H., Fong, D. Y. T., & Auyeung, M. (2020). Effects of spiritual resilience on psychological distress and health-related quality of life in Chinese people with Parkinson’s disease. Quality of Life Research, 29(11), 3065–3073. https://doi.org/10.1007/s11136-020-02562-x
Lou, J. S. (2015). Fatigue in Parkinson’s disease and potential interventions. NeuroRehabilitation, 37(1), 25–34. https://doi.org/10.3233/NRE-151238
Sanchez-Luengos, I., Balboa-Bandeira, Y., Lucas-Jiménez, O., Ojeda, N., Peña, J., & Ibarretxe-Bilbao, N. (2021). Effectiveness of cognitive rehabilitation in Parkinson’s disease: A systematic review and meta-analysis. Journal of Personalized Medicine. https://doi.org/10.3390/jpm11050429
Schulz, G. M., & Grant, M. K. (2000). Effects of speech therapy and pharmacologic and surgical treatments on voice and speech in Parkinson’s disease: A review of the literature. Journal of Communication Disorders, 33(1), 59–88. https://doi.org/10.1016/S0021-9924(99)00025-8
Santos-Garcia, D., Deus-Fonticoba, T., Cores, C., Muñoz, G., Paz-González, J. M., Martinez-Miró, C., et al. (2021). Predictors of clinically significant quality of life impairment in Parkinson’s disease. NPJ Parkinson’s Disease. https://doi.org/10.1038/s41531-021-00256-w
van Wamelen, D. J., Sauerbier, A., Leta, V., Rodriguez-Blazquez, C., Falup-Pecurariu, C., Rodriguez-Violante, M., & Chaudhuri, K. R. (2021). Cross-sectional analysis of the Parkinson’s disease Non-motor International Longitudinal Study baseline non-motor characteristics, geographical distribution and impact on quality of life. Scientific Reports, 11(1), 1–11. https://doi.org/10.1038/s41598-021-88651-4
Kwok, J. J. Y. Y., Auyeung, M., & Chan, H. Y. L. (2020). Examining factors related to health-related quality of life in people with Parkinson’s disease. Rehabilitation Nursing, 45(3), 122–130. https://doi.org/10.1097/rnj.0000000000000179
Acknowledgements
The authors would like to thank ASPARBI, ASOPARA and all of the participants involved in the study as well as the English language editing service.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study was supported by BBK foundation (P201902032-12/1284), the Department of Education of the Basque Government (IT946-16) and Research Staff Training Programme Grant from the Basque Government (PRE_2018_1_0379).
Author information
Authors and Affiliations
Contributions
NI-B, JP, NO, and OL-J contributed to the study design, conceptualization, and implementation. IS-L and JP conducted statistical analyses and interpretation. JCG-E, MAG-B, RV-P, NF-B, and IS-L contributed to recruitment and data collection. IS-L, OL-J, and NI-B carried out drafting. All authors contributed to the writing and revision of the final manuscript. All authors have approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare no competing interests.
Ethical approval
The study protocol is part of a larger study approved by the Ethics Committee of the University of Deusto (ETK-20/15-16) and the Research Ethics Committee of Basque Country (CEIm-E) (PI2018147).
Consent to participate
In accordance with the Declaration of Helsinki, all participants were volunteers and signed an informed consent to participate in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sanchez-Luengos, I., Lucas-Jiménez, O., Ojeda, N. et al. Predictors of health-related quality of life in Parkinson’s disease: the impact of overlap between health-related quality of life and clinical measures. Qual Life Res 31, 3241–3252 (2022). https://doi.org/10.1007/s11136-022-03187-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-022-03187-y