Abstract
Purpose
Frailty is an important predictor of adverse health events in older people, and improving quality of life (QOL) is increasingly recognised as a focus for services in this population. This systematic review synthesised evidence of the relationship between frailty and QOL in community-dwelling older people, with an emphasis on how this relationship varied across QOL domains.
Methods
We conducted a systematic review with meta-analysis. We searched five databases for reports of QOL in older people with frailty and included studies based on pre-defined criteria. We conducted meta-analyses comparing “frail” and “not frail” groups for each QOL scale where data were available. We compared pooled results to distribution-based and known-group differences to enhance interpretation. We summarised reported cross-sectional and longitudinal analyses.
Results
Twenty-two studies (24,419 participants) were included. There were medium or larger standardised mean differences for 24 of 31 QOL scales between frail and not frail groups, with worse QOL for frail groups. These scales encompassed constructs of health-related quality of life as well as psychological and subjective well-being. There were similar findings from mean difference meta-analyses and within-study analyses.
Conclusions
The association between frailty and lower QOL across a range of constructs is clear and often substantial. Future research should establish whether causal mechanisms link the constructs, which aspects of QOL are most important to older people with frailty, and investigate their tractability. Services focused on measuring and improving QOL for older people with frailty should be introduced.
Similar content being viewed by others
Introduction
Enhancing Quality of Life (QOL) has been an explicit or implicit goal for individuals, communities, nations and the world [1]. QOL is a complex concept and its precise formulation is contested [1,2,3]. It is defined by the World Health Organization (WHO) as: “An individual’s perceptions of their position in life, in the context of the culture and value systems in which they live, and in relation to their goals, expectations, standards and concerns” [4]. The global demographic transition to older populations has meant health care organisations internationally have adopted a greater focus on enhancing QOL for older people [5]. Indeed, prioritising QOL in later life, in preference to disease-based outcomes, is consistent with the views of older people themselves [6, 7]. QOL measures can help estimate the needs of a population and improve clinical decision making, resource allocation and policy [8,9,10], and QOL assessments are increasingly collected in studies involving older people [11,12,13].
The concept of frailty as an abnormal health state characterised by loss of biological reserves related to the aging process has emerged in the last 15 years. It has proved a better discriminator than chronological age in the prediction of mortality and variations in outcomes in later life [14], and robust models have been developed and validated to identify frailty [15]. Approximately, one in ten people over 65 years, and between a quarter and a half of those aged over 85 years, are living with frailty [15]. There is an argument that frailty should be considered and managed as a long-term condition [16] and assessments for frailty are increasingly being incorporated into routine practice to ensure both its improved detection and the subsequent delivery of care that gives greater emphasis to QOL for older people living with frailty.
The relationship between frailty and QOL has attracted research interest, though the findings have been inconsistent. A systematic review reported an inverse relationship between frailty and QOL among community-dwelling older people [17]. However, there were limitations with the review, notably the limited use of meta-analysis, little consideration of differing constructs of QOL and inclusion of data from intervention studies, which are prone to selection bias, producing unrepresentative samples [18]. We have therefore conducted a further systematic review to investigate the impact of frailty on QOL, and vice versa, but with a particular focus on the domains of QOL that are most affected. We anticipate that this information will facilitate more targeted approaches for interventions for older people with frailty.
Methods
Study inclusion criteria
We conducted a systematic review with meta-analysis. We included cohort or cross-sectional studies. Studies where the data were part of an intervention study, even if collected prior to intervention, were excluded. We included studies if the participants were community-dwelling older people (mean age ≥ 65 years). We included studies that reported QOL by frailty status, or association between frailty and QOL.
We included studies with a validated instrument for frailty. Because of the diverse definitions for QOL, we included studies with instruments which were described as measuring “quality of life”, “well-being” or “life satisfaction” by a study that met the other inclusion criteria, or where this was implied by the name of the instrument itself, such as The World Health Organization Quality of Life (WHOQOL) scale [4].
We only included studies with reports written in English, or where authors could provide data in a format we could utilise.
Search strategy
We developed a search strategy with an information specialist using controlled vocabulary and text words to search databases including AMED, CINAHL, MEDLINE and Web of Science (last searched 4 April 2017; see Online Resource 1).
We adopted an iterative search procedure, updating our strategy and repeating our searches to incorporate specific terms for each instrument that at least one study identified as a measure of QOL, to ensure that we identified and pooled all available data.
Data collection
Two reviewers independently conducted each stage of study selection, data extraction and assessment of risk of bias and compared results. Disagreements were resolved by discussion with other members of the review team. Study authors were contacted for further information where necessary.
Selection of studies
We assessed the titles and abstracts from the electronic searches against the stated eligibility criteria. We obtained full text articles of potentially eligible studies and assessed these against the criteria to determine study inclusion. This process was repeated each time additional QOL instruments were identified.
Data extraction and management
We extracted data using a pre-specified and piloted form (see Online Resource 2 for template of electronic form). Where scores for a particular instrument were reported inconsistently, we standardised their scaling (e.g. transforming WHOQOL-BREF scores to 0–100).
Risk of bias in individual studies
We assessed risk of bias at the study level using the modified Newcastle–Ottawa Scale [19, 20]. Studies were assessed on the domains of selection, comparability, exposure (i.e. frailty) and outcome (i.e. QOL). Outcome assessment scored one star for self-report because of the appropriateness of this for QOL measurement. The maximum achievable score (least risk of bias) was eight stars. Scores of five stars or more were considered moderate to good quality, although studies were incorporated in the synthesis regardless of rating [20].
Data synthesis
We calculated and pooled standardised mean differences (SMDs) for each QOL scale using inverse-variance random-effects meta-analysis, where feasible. We grouped participants as “frail” versus “not frail” for these purposes; where other groupings were given, we assigned moderate and severe frailty as “frail” and fit, robust, pre-frail, vulnerable and mild frailty as “not frail”. We pooled mean differences (MDs) for each QOL scale where data were reported in multiple studies following the approach above. Meta-analyses were conducted in Review Manager 5.3 [21].
QOL instruments have a variety of score ranges. Even when ranges are consistent, scores on different scales cannot usually be meaningfully compared, including subscales of the same instrument. Additionally, minimal clinically important differences (MCIDs) are not well established. Therefore, to aid interpretation, we have (1) described SMDs using standard rules of thumb for effect sizes (e.g. small, medium, large; detailed in Online Resource 3) [22]; (2) compared pooled MDs with reference values calculated from general populations in large-scale studies where possible (see Online Resource 3).
We summarised cross-sectional and longitudinal analyses reported in included studies of association between QOL and frailty measures with a narrative synthesis.
Results
Study selection
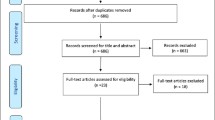
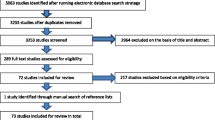
See Fig. 1 for the study selection process. We identified 4537 records through database searches and 24 additional references from other systematic reviews. Twenty-one groups of participants were included in the review, reported in 30 articles [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52]. For one group of participants (the English Longitudinal Study of Aging [ELSA] cohort), we present the results as two separate studies, as differing frailty instruments, age limits and timepoints were used [29, 30]. Therefore, 22 studies were included in the review [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44].
Study characteristics
Study characteristics are presented in Table 1.
Design
Five studies presented longitudinal analyses incorporating QOL and frailty data [23, 24, 29, 32, 42]. The remaining studies presented only cross-sectional data or analyses for QOL and frailty variables. Nineteen studies examined the relationship between frailty and QOL [23,24,25,26,27, 29, 30, 32,33,34,35,36,37,38,39,40,41,42, 44], two studies assessed the psychometric properties of a frailty instrument [28, 31] and one study assessed the psychometric properties of a QOL instrument [43].
Setting
Data were collected from populations in 12 countries across Europe, Asia and North and South America. The first wave of data was collected in the 1990s by one study [24], in the 2000s by ten studies [25, 29, 30, 32,33,34,35,36, 38, 39] and in the 2010s by seven studies [23, 26, 28, 31, 37, 43, 44], with four studies not reporting date of data collection [27, 40,41,42].
Participants
The included studies report data from 24,419 participants in total (median: 479 participants; smallest study, 95 [41]; largest study, 5703 [24]; not double-counting ELSA cohort participants). The overall mean age (composite standard deviation) was 76.1 (7.5) years where such data were provided. There were 13,905 female participants (57%; study range 33–82%).
Most studies recruited participants from open adverts or mailouts; five studies recruited directly through health services [25, 26, 35, 37, 43].
Frailty ascertainment
Frailty status within studies was ascertained using: Fried phenotype criteria (eight studies [26, 29, 34, 36,37,38,39,40]; Tilburg Frailty Indicator (four [28, 31,32,33]); cumulative deficit model (three [24, 30, 44]); Study of Osteoporotic Fractures criteria (two [25, 27]); Groningen Frailty Indicator (two [23, 41]); the Chinese Canadian study of health and aging clinical frailty scale [43]; the Hebrew Rehabilitation Center for Aged Vulnerability Index and the Vulnerable Elders Survey [35] and the Brief frailty measure and Frailty Index [42].
QOL instruments
Descriptions of the QOL instruments used in the studies are provided in Online Resource 4. Twenty studies reported QOL estimates based on a single instrument only; two studies used more than one instrument [28, 31]. Eight of the instruments have multiple scales (i.e. dimensions).
QOL within studies was measured using: Medical Outcomes Study 36-item Short-Form health survey (SF-36, five studies [26, 34, 37,38,39]); World Health Organization’s Quality of Life short-form instrument (WHOQOL-BREF; four [27, 32, 33, 35]); Control, Autonomy, Self-realisation and Pleasure, 19-item questionnaire (CASP-19; three [29, 30, 43]); European Health Information Surveys project 8-item QOL index (EUROHIS-QOL; two [28, 31]); 12-item Short-Form survey (SF-12) [31]; Older Adults WHOQOL module (WHOQOL-OLD) [28]; 18-item version of Ryff’s Psychological Well-Being scale [24]; Older People’s Quality of Life Questionnaire (OPQOL) [25]; Satisfaction with Life Scale (SWLS) [41]; EuroQol visual analogue scale (EQ-VAS) [36]; a modified Life-Satisfaction Terrible-Delightful scale [42] and three unreferenced instruments: a single-item evaluation of QOL [23], and two different multi-item life satisfaction instruments [40, 44].
Risk of bias within studies
The median score of the modified Newcastle–Ottawa Scale was 5 (range 2 to 6). Most studies recruited broad, representative samples, but few provided sample size calculations or described comparability with non-respondents. Most studies provided adjustment for relevant factors and conducted appropriate analyses that were sufficiently reported.
Synthesis of results
Standardised mean difference meta-analyses
Random-effects meta-analyses estimated statistically significant SMDs in favour of the ‘not frail’ group for 27 of 31 scales, of which the SMD size were: very large for the WHOQOL physical domain (Fig. 2k), large for 13 scales (SF-36 Physical functioning, SF-36 Social functioning, SF-36 Physical Component Summary, WHOQOL-BREF Psychological, WHOQOL-BREF Environment, CASP-19 Total, CASP-19 Autonomy, CASP-19 C + A + S [eudaimonic], OPQOL Total, OPQOL Health, OPQOL Independence, EQ-VAS, SWLS; Fig. 2a, f, i, l, n, o, q, s, u, w, y, δ and ε), medium for ten scales and small for three scales (forest plots in Fig. 2, additional data in Online Resource 5) [22]. Among the other four scales, SMDs were small for one scale (Fig. 2r), very small for two scales (Fig. 2x and β) and small favouring people with frailty for the CASP-19 control scale (Fig. 2p). Estimates of SMD were insufficiently precise for the upper and lower bounds of 95% confidence intervals to have the same rule of thumb interpretation except for one scale (Fig. 2t, CASP-19 pleasure scale, medium difference).
There was evidence of substantial heterogeneity between studies (I2 > 20%) for 14 of the 16 scales with more than one study contributing to the meta-analysis. Additionally, there was evidence of substantial heterogeneity between QOL scales (I2 = 78%). Due to the limited number of studies contributing to each QOL scale and many plausible sources of heterogeneity, we did not investigate heterogeneity through subgroup analyses.
Mean difference meta-analyses
Pooled results of studies reporting the SF-36 and WHOQOL-BREF found clinically and statistically significant differences between frail and not frail groups for each QOL scale. Forest plots and data tables for MD meta-analyses are presented in Online Resource 6.
Individual study cross-sectional analyses
Measures and analyses from individual studies are reported in Online Resource 7.
Fourteen studies reported on statistical significance of bivariate associations between measures of frailty and QOL. These were statistically significant (p < 0.05) for all reported analyses in eleven studies [26,27,28,29, 31, 32, 39,40,41,42,43], but only for some QOL scales in the other three studies’ analyses [25, 34, 37].
In 13 studies [24,25,26,27, 30, 34,35,36, 38,39,40, 42, 44], there were multivariate cross-sectional analyses, with statistically significant associations between frailty and QOL in 44 of the 58 analyses. Those analyses with non-significant results were characterised by small numbers of participants and large numbers of additional variables suggesting they may have been over-adjusted [24, 27, 34, 35].
Longitudinal analyses
Four studies reported longitudinal analyses. In Gale 2014, 4-year frailty was associated with baseline CASP-19 total, hedonic and eudaimonic scores in a model adjusted for variables including baseline frailty and depressive symptoms [relative risk of frailty (95% CI) 0.62 (0.52 to 0.74); 0.70 (0.59 to 0.82) and 0.64 (0.53 to 0.76) per SD increase in respective CASP-19 scores] [29]. However, in the Canadian Study of Health and Aging, 5-year frailty was not associated with Ryff’s psychological well-being scale; associations were mediated via depression [52]. Gobbens 2012 reported that addition of baseline frailty to a multivariable model explained an additional 3.7%, 4.4%, 4.6% and 1.8% of the variance in 2-year physical, psychological, social and environmental WHOQOL scores, respectively [32]. Furthermore, Gale 2014 reported that reduced 4-year CASP-19 scores were associated with increased incidence of pre-frailty and frailty among those fit at baseline in adjusted models [29]. According to St John 2013, 5-year life satisfaction domains and life satisfaction overall were explained by baseline frailty status, although unlike other analyses presented here the model was not adjusted for baseline values of the outcome variable nor were the results significant for the life satisfaction domains of housing and self-esteem [42].
Risk of bias across studies
Visual inspection of funnel plots revealed no evidence of asymmetry, indicating no evidence of publication bias.
Discussion
To investigate the QOL of community-dwelling older people with frailty, we systematically reviewed the literature and identified 22 observational studies (24,419 participants) that met our broad inclusion criteria. Evidence indicates that people with frailty have worse QOL than people without frailty, with medium to large differences between the groups. This association is robust to adjustment for relevant variables such as age, gender and depression.
QOL is a complex concept and we anticipated diversity in the instruments used in the included studies. Fourteen instruments were used in the 22 studies, many with multiple scales that targeted a wide range of constructs including those focused on health (e.g. limitations in activities, pain and mental health) as well as broader conceptions of well-being such as psychological well-being (e.g. sense of control and self-acceptance); satisfaction with relationships or circumstances (e.g. housing, finances or transport) and overall life-satisfaction and QOL. Our findings therefore relate to QOL as a broadly defined concept and imply that it is a valid outcome for the attention of service providers and researchers in relation to older people with frailty. Future studies should explain their choice of instrument and the importance of the embedded constructs to people with frailty. Ideally, older people with frailty should be involved in instrument selection.
While the point estimate of SMD was medium to large for many QOL scales, there were some notable exceptions and patterns. Physical functioning and satisfaction with health scales were among those with the largest difference (in particular WHOQOL-BREF Physical, SF-36 Physical functioning, OPQOL Health and EQ-VAS), which is perhaps unsurprising given the conceptual overlap with frailty. More broadly, total scores of the OPQOL, CASP-19 and SWLS also had large differences. However, there were inconsistent results for social scales, with the OPQOL Social scale being non-significant and its confidence interval not overlapping those of either the SF-36 Social functioning (large SMD) or WHOQOL-BREF Social (medium SMD) scales. Similarly, there are divergent results for the conceptually similar WHOQOL-BREF Environment scale (large SMD) and the OPQOL Home and Finances scales (small/non-significant SMDs). While there are some differences in focus between the instruments, it would be useful to see if the OPQOL results (currently based on a single study) are repeated, and if so whether it points to aspects with differing importance or perception between people with and without frailty, or perhaps whether it is indicative of problematic scales.
This systematic review updates the earlier review [17] and includes eleven additional studies [23, 27, 28, 34,35,36,37, 40,41,42, 44]. By using an SMD approach, we were able to compare across QOL scales, including a greater number in meta-analyses than the previous review which included only the SF-36 PCS and MCS. The method of calculating the PCS and MCS can lead to anomalous results such as higher MCS in people with lower physical and mental subscales due to the inclusion of all eight SF-36 subscales and the use of negative weights in calculation of each summary score, meaning they should be interpreted in conjunction with the subscales [53, 54]. Our synthesis enabled this, allowing the identification of small to large SMDs across these health-related subscales. We were also able to identify a relatively consistent effect across other QOL constructs. However, our analyses were limited by dichotomising the whole population as ‘frail’ or ‘not frail’, rather than including a pre-frail category, which would have necessitated only examining portions of the population in each analysis or double-counting.
There was a lack of conceptual clarity in some of the studies, as their frailty and QOL definitions overlapped substantially. For example, the Fried criteria of exhaustion, low energy expenditure, slow walking speed and weak grip strength has much in common with the vitality and physical functioning subscales of the SF-36. Similarly, two studies used the Tilburg Frailty Index in conjunction with the WHOQOL-BREF, which contain similar domains. Nevertheless, other studies identified associations across distinct constructs such as between a cumulative deficit model of frailty and the CASP-19.
We were limited in the longitudinal data that were available from the studies, which limited our ability to examine causality. However, there is some evidence that lower psychological well-being may cause incident frailty and that frailty may cause reductions in multiple QOL domains. Evidence of a bi-directional relationship should be treated with caution at this stage. Future research should explore the relationship between QOL overall, factors that contribute to QOL, well-specified models of frailty and proposed mechanisms linking QOL and frailty [e.g. 55–58] using panel data with multiple time points in multilevel models to help disentangle the associations identified in this systematic review. Research such as this would enable better understanding of whether, for example, protecting psychological well-being may lessen frailty, and whether combating frailty could improve QOL. Experimental research is also required to investigate the extent to which QOL is a tractable outcome for this population.
There has been half a century of conceptual thinking and field research about QOL and over two decades relating to frailty. It is now more widely recognised that modern medical interventions applied to older people with frailty can result in unintended harms rather than benefits [15]. Indeed, simply applying standard long-term condition guidelines leads to excessive treatment burdens for older people with multiple chronic conditions [59]; a situation that is commonplace in later life [60]. New care paradigms for older people with frailty are being advanced in Europe [61] and elsewhere [62] in which there is a reframing of service goals and outcomes with a greater emphasis on individualised, person-centred approaches. In the future, health services for older people with frailty will extend the traditional medical approaches to address more of the things that matter to older people [7] and emphasise linking the person to their local community [63].
The findings from our review are therefore reassuring: frailty and QOL are negatively associated with large differences by frailty status for a wide range of QOL constructs. This is important for research funders and service planners who should feel confident to commission, design and introduce novel services with an explicit focus on measuring and improving QOL outcomes for older people with frailty.
References
Costanza, R., Fisher, B., Ali, S., Beer, C., Bond, L., Boumans, R., et al. (2007). Quality of life: An approach integrating opportunities, human needs, and subjective well-being. Ecological Economics, 61, 267–276. https://doi.org/10.1016/j.ecolecon.2006.02.023.
Galloway, S., Bell, D., Hamilton, C., & Scullion, A. (2006). Well-being and quality of life: Measuring the benefits of culture and sport-a literature review and thinkpiece. Edinburgh: Scottish Executive: Social Research).
Andelman, R., Board, R., Carman, L. L., Cummins, R. A., Ferriss, A., Friedman, P., et al. (1998). Quality of life definition and terminology: A discussion document from the International Society for Quality of Life Studies: The International Society for Quality-of-Life Studies (ISQOLS).
WHOQOL Group. (1993). Study protocol for the World Health Organization project to develop a Quality of Life assessment instrument (WHOQOL). Quality of life Research, 2(2), 153–159. https://doi.org/10.1007/BF00435734.
World Health Organization. (2015). World report on ageing and health. Geneva: World Health Organization.
Gabriel, Z., & Bowling, A. (2004). Quality of life from the perspectives of older people. Ageing and Society, 24(5), 675–691. https://doi.org/10.1017/s0144686x03001582.
National Voices, Age, U. K. & UCL Partners (2014). I’m still me: A narrative for coordinated support for older people: National Voices. Retrieved September 4, 2018 from https://www.nationalvoices.org.uk/publications/our-publications/im-still-me.
Arnold, S. B. (1991). Measurement of quality of life in the frail elderly. In J. Birren, J. Lubben, J. Rowe & D. Deutchman (Eds.), The concept and measurement of quality of life in the frail elderly (pp. 50–73). London: Academic Press.
McGregor, J. A., Camfield, L., & Woodcock, A. (2009). Needs, wants and goals: Wellbeing, quality of life and public policy. Applied research in Quality of Life, 4(2), 135–154. https://doi.org/10.1007/s11482-009-9069-7.
Huber, M., Knottnerus, J. A., Green, L., van der Horst, H., Jadad, A. R., Kromhout, D., et al. (2011). How should we define health? BMJ, https://doi.org/10.1136/bmj.d4163.
Netuveli, G., Wiggins, R. D., Hildon, Z., Montgomery, S. M., & Blane, D. (2006). Quality of life at older ages: Evidence from the English longitudinal study of aging (wave 1). Journal of Epidemiology & Community Health, 60(4), 357–363. https://doi.org/10.1136/jech.2005.040071.
Layte, R., Sexton, E., & Savva, G. (2013). Quality of life in older age: Evidence from an Irish cohort study. Journal of the American Geriatrics Society, 61(Suppl 2), S299–S305. https://doi.org/10.1111/jgs.12198.
Clegg, A., Barber, S., Young, J., Forster, A., & Iliffe, S. (2011). The Home-Based Older People’s Exercise (HOPE) trial: Study protocol for a randomised controlled trial. Trials, 12(1), 143. https://doi.org/10.1186/1745-6215-12-143.
Song, X., Mitnitski, A., & Rockwood, K. (2010). Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. Journal of the American Geriatrics Society, 58(4), 681–687. https://doi.org/10.1111/j.1532-5415.2010.02764.x.
Clegg, A., Young, J., Iliffe, S., Rikkert, M. O., & Rockwood, K. Frailty in elderly people. The Lancet. 381(9868), 752–762, https://doi.org/10.1016/S0140-6736(12)62167-9.
Harrison, J. K., Clegg, A., Conroy, S. P., & Young, J. (2015). Managing frailty as a long-term condition. Age and Ageing, 44(5), 732–735. https://doi.org/10.1093/ageing/afv085.
Kojima, G., Iliffe, S., Jivraj, S., & Walters, K. (2016). Association between frailty and quality of life among community-dwelling older people: A systematic review and meta-analysis. Journal of Epidemiology & Community Health, 70(7), 716–721. https://doi.org/10.1136/jech-2015-206717.
Kennedy-Martin, T., Curtis, S., Faries, D., Robinson, S., & Johnston, J. (2015). A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials, 16, 495. https://doi.org/10.1186/s13063-015-1023-4.
Wells, G., Shea, B., O’Connell, D., Peterson, J., Welch, V., Losos, M., et al. (2014). The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Retrieved April 4, 2018 from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Alshabanat, A., Zafari, Z., Albanyan, O., Dairi, M., & FitzGerald, J. M. (2015). Asthma and COPD Overlap Syndrome (ACOS): A systematic review and meta analysis. PLoS ONE, 10(9), e0136065. https://doi.org/10.1371/journal.pone.0136065.
. Review Manager (RevMan) (2014). (5.3 ed.). Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration.
Sawilowsky, S. S. (2009). New effect size rules of thumb. Journal of Modern Applied Statistical Methods. https://doi.org/10.22237/jmasm/1257035100.
Ament, B. H. L., de Vugt, M. E., Verhey, F. R. J., & Kempen, G. I. J. M. (2014). Are physically frail older persons more at risk of adverse outcomes if they also suffer from cognitive, social, and psychological frailty? European Journal of Ageing, 11(3), 213–219. https://doi.org/10.1007/s10433-014-0308-x.
Bilotta, C., Bowling, A., Case, A., Nicolini, P., Mauri, S., Castelli, M., et al. (2010). Dimensions and correlates of quality of life according to frailty status: A cross-sectional study on community-dwelling older adults referred to an outpatient geriatric service in Italy. Health and Quality of Life Outcomes. 8, 56, https://doi.org/10.1186/1477-7525-8-56.
Andrew, M. K., Fisk, J. D., & Rockwood, K. (2012). Psychological well-being in relation to frailty: A frailty identity crisis? Int Psychogeriatr, 24(8), 1347–1353. https://doi.org/10.1017/S1041610212000269.
Chang, Y.-W., Chen, W.-L., Lin, F.-G., Fang, W.-H., Yen, M.-Y., Hsieh, C.-C., et al. (2012). Frailty and its impact on health-related quality of life: A cross-sectional study on elder community-dwelling preventive health service users. PLoS ONE, 7(5), e38079. https://doi.org/10.1371/journal.pone.0038079.
Chang, S. F., & Wen, G. M. (2016). Association of frail index and quality of life among community-dwelling older adults. Journal of Clinical Nursing, 25, 2305–2316. https://doi.org/10.1111/jocn.13248.
Coelho, T., Santos, R., Paul, C., Gobbens, R. J., & Fernandes, L. (2015). Portuguese version of the Tilburg Frailty Indicator: Transcultural adaptation and psychometric validation. Geriatrics & Gerontology International, 15, 951–960. https://doi.org/10.1111/ggi.12373.
Gale, C. R., Cooper, C., Deary, I. J., & Aihie Sayer, A. (2014). Psychological well-being and incident frailty in men and women: The English Longitudinal Study of Ageing. Psychological Medicine, 44(4), 697–706. https://doi.org/10.1017/S0033291713001384.
Hubbard, R. E., Goodwin, V. A., Llewellyn, D. J., Warmoth, K., & Lang, I. A. (2014). Frailty, financial resources and subjective well-being in later life. Archives of Gerontology & Geriatrics, 58(3), 364–369. https://doi.org/10.1016/j.archger.2013.12.008.
Freitag, S., Schmidt, S., & Gobbens, R. (2016). Tilburg frailty indicator: German translation and psychometric testing. Zeitschrift fur Gerontologie und Geriatrie, 49, 86–93. https://doi.org/10.1007/s00391-015-0889-9.
Gobbens, R. J. J., van Assen, M. A. L. M., Luijkx, K. G., & Schols, J. M. G. A. (2012). The predictive validity of the tilburg frailty indicator: Disability, health care utilization, and quality of life in a population at risk. Gerontologist, 52, 619–631. https://doi.org/10.1093/geront/gnr135.
Gobbens, R. J. J., Luijkx, K. G., & van Assen, M. A. L. M. (2013). Explaining quality of life of older people in the Netherlands using a multidimensional assessment of frailty. Quality of Life Research, 22(8), 2051–2061. https://doi.org/10.1007/s11136-012-0341-1.
Jurschik, P., Nunin, C., Botigue, T., Escobar, M. A., Lavedan, A., & Viladrosa, M. (2012). Prevalence of frailty and factors associated with frailty in the elderly population of Lleida, Spain: The FRALLE survey. Archives of Gerontology & Geriatrics, 55(3), 625–631. https://doi.org/10.1016/j.archger.2012.07.002.
Kanauchi, M., Kubo, A., Kanauchi, K., & Saito, Y. (2008). Frailty, health-related quality of life and mental well-being in older adults with cardiometabolic risk factors. International Journal of Clinical Practice, 62(9), 1447–1451. https://doi.org/10.1111/j.1742-1241.2008.01830.x.
Lahousse, L., Maes, B., Ziere, G., Loth, D. W., Verlinden, V. J., Zillikens, M. C., et al. (2014). Adverse outcomes of frailty in the elderly: The Rotterdam Study. European Journal of Epidemiology, 29(6), 419–427. https://doi.org/10.1007/s10654-014-9924-1.
Lenardt, M. H., Kolb Carneiro, H., Albino, N., J., & Willig, H., M (2014). Quality of life of frail elderly users of the primary care. Acta Paulista de Enfermagem, 27, 399–404. https://doi.org/10.1590/1982-0194201400067.
Lin, C. C., Li, C. I., Chang, C. K., Liu, C. S., Lin, C. H., Meng, N. H., et al. (2011). Reduced health-related quality of life in elders with frailty: A cross-sectional study of community-dwelling elders in Taiwan. PLoS ONE. 6(7), E21841, https://doi.org/10.1371/journal.pone.0021841.
Masel, M. C., Graham, J. E., Reistetter, T. A., Markides, K. S., & Ottenbacher, K. J. (2009). Frailty and health related quality of life in older Mexican Americans. Health and Quality of Life Outcomes, 7, 70. https://doi.org/10.1186/1477-7525-7-70.
Pinto, J. M., Fontaine, A. M., & Neri, A. L. (2016). The influence of physical and mental health on life satisfaction is mediated by self-rated health: A study with Brazilian elderly. Archives of Gerontology and Geriatrics, 65, 104–110. https://doi.org/10.1016/j.archger.2016.03.009.
Simone, P. M., & Haas, A. L. (2013). Frailty, leisure activity and functional status in older adults: Relationship with subjective well being. Clinical Gerontologist: The Journal of Aging and Mental Health, 36(4), 275–293. https://doi.org/10.1080/07317115.2013.788114.
St John, P. D., Tyas, S. L., & Montgomery, P. R. (2013). Life satisfaction and frailty in community-based older adults: Cross-sectional and prospective analyses. International Psychogeriatrics, 25(10), 1709–1716. https://doi.org/10.1017/S1041610213000902.
Wu, T.-Y., Chie, W.-C., Kuo, K.-L., Wong, W.-K., Liu, J.-P., Chiu, S.-T., et al. (2013). Quality of life (QOL) among community dwelling older people in Taiwan measured by the CASP-19, an index to capture QOL in old age. Archives of Gerontology and Geriatrics, 57(2), 143. https://doi.org/10.1016/j.archger.2013.03.010.
Yang, F., Gu, D., & Mitnitski, A. (2016). Frailty and life satisfaction in Shanghai older adults: The roles of age and social vulnerability. Archives of Gerontology & Geriatrics, 67, 68–73. https://doi.org/10.1016/j.archger.2016.07.001.
Bilotta, C., Bowling, A., Nicolini, P., Casè, A., Pina, G., Rossi, S. V., et al. (2011). Older People’s Quality of Life (OPQOL) scores and adverse health outcomes at a one-year follow-up. A prospective cohort study on older outpatients living in the community in Italy. Health & Quality of Life Outcomes. https://doi.org/10.1186/1477-7525-9-72.
Gobbens, R. J., & van Assen, M. A. (2014). The prediction of quality of life by physical, psychological and social components of frailty in community-dwelling older people. Quality of Life Research, 23(8), 2289–2300. https://doi.org/10.1007/s11136-014-0672-1.
Gobbens, R. J., van Assen, M. A., Luijkx, K. G., & Schols, J. M. (2012). Testing an integral conceptual model of frailty. Journal of Advanced Nursing, 68(9), 2047–2060. https://doi.org/10.1111/j.1365-2648.2011.05896.x.
Gobbens, R. J., & van Assen, M. A. (2016). Psychometric properties of the Dutch WHOQOL-OLD. Health & Quality of Life Outcomes, 14, 103. https://doi.org/10.1186/s12955-016-0508-5.
Lenardt, M. H., Kolb Carneiro, N. H., Binotto, M. A., Hautsch Willig, M., Lourenço, T. M., & Albino, J. (2016). Frailty and quality of life in elderly primary health care users. Revista Brasileira de Enfermagem, 69, 448–453. https://doi.org/10.1590/0034-7167.2016690309i.
Li, C. I., Lin, C. H., Lin, W. Y., Liu, C. S., Chang, C. K., Meng, N. H., et al. (2014). Successful aging defined by health-related quality of life and its determinants in community-dwelling elders. BMC Public Health, 14, 1013. https://doi.org/10.1186/1471-2458-14-1013.
Masel, M. C., Ostir, G. V., & Ottenbacher, K. J. (2010). Frailty, mortality, and health-related quality of life in older Mexican Americans. Journal of the American Geriatrics Society, 58(11), 2149–2153. https://doi.org/10.1111/j.1532-5415.2010.03146.x.
Rao, S., Wallace, L., Theou, O., & Rockwood, K. (2017). Is it better to be happy or not depressed? Depression mediates the effect of psychological well-being on adverse health outcomes in older adults. International Journal of Geriatric Psychiatry, 32(9), 1000–1008. https://doi.org/10.1002/gps.4559.
Taft, C., Karlsson, J., & Sullivan, M. (2001). Do SF-36 summary component scores accurately summarize subscale scores? Quality of Life Research, 10(5), 395–404. https://doi.org/10.1023/A:1012552211996.
Ware, J. E., & Kosinski, M. (2001). Interpreting SF-36 summary health measures: A response. Quality of Life Research, 10(5), 405–413. https://doi.org/10.1023/A:1012588218728. discussion 415–420.
Bowling, A., & Grundy, E. (2009). Differentials in mortality up to 20 years after baseline interview among older people in East London and Essex. Age and Ageing, 38(1), 51–55. https://doi.org/10.1093/ageing/afn220.
Espinoza, S. E., & Fried, L. P. (2007). Risk factors for frailty in the older adult. Clinical Geriatrics, 15(6), 37–44.
Fillit, H., & Butler, R. N. (2009). The frailty identity crisis. Journal of the American Geriatrics Society, 57(2), 348–352. https://doi.org/10.1111/j.1532-5415.2008.02104.x.
Rizzoli, R., Reginster, J. Y., Arnal, J. F., Bautmans, I., Beaudart, C., Bischoff-Ferrari, H., et al. (2013). Quality of life in sarcopenia and frailty. Calcified Tissue International, 93(2), 101–120. https://doi.org/10.1007/s00223-013-9758-y.
Hughes, L. D., McMurdo, M. E. T., & Guthrie, B. (2013). Guidelines for people not for diseases: The challenges of applying UK clinical guidelines to people with multimorbidity. Age and Ageing, 42(1), 62–69. https://doi.org/10.1093/ageing/afs100.
Barnett, K., Mercer, S. W., Norbury, M., Watt, G., Wyke, S., & Guthrie, B. (2012). Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. The Lancet, 380(9836), 37–43. https://doi.org/10.1016/S0140-6736(12)60240-2.
ADVANTAGE Joint Action (2018). State of the art report on the prevention and management of frailty: ADVANTAGE Joint Action. Retrieved September 4, 2018 from http://advantageja.eu/images/SoAR-AdvantageJA_Fulltext.pdf.
Canadian Frailty Network (2018). Canadian Frailty Network | Geriatric research. Retrieved September 4, 2018 from http://www.cfn-nce.ca/.
Rippon, S., & Hopkins, T. (2015). Head, hands and heart: asset-based approaches in health care. A review of the conceptual evidence and case studies of asset-based approaches in. In Health, care and wellbeing. London: The Health Foundation.
Acknowledgements
The authors acknowledge Anne Heaven, who helped to conceive the study, and University of Leeds librarians Deirdre Andre and Emily Wheeler, who helped to develop and execute the searches. Three of the authors of this paper (LB, AC, JY) were supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) Yorkshire and Humber. http://www.clahrc-yh.nihr.ac.uk. The views and opinions expressed are those of the authors, and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. Other authors contributed from within their existing roles. No sponsors had involvement in the study design, data collection, analysis or interpretation, report writing or decision to submit the article for publication.
Funding
This study was partly funded by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) Yorkshire and Humber. http://www.clahrc-yh.nihr.ac.uk.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Crocker, T.F., Brown, L., Clegg, A. et al. Quality of life is substantially worse for community-dwelling older people living with frailty: systematic review and meta-analysis. Qual Life Res 28, 2041–2056 (2019). https://doi.org/10.1007/s11136-019-02149-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-019-02149-1