Abstract
Purpose
Huntington disease (HD) is an incurable terminal disease. Thus, end of life (EOL) concerns are common in these individuals. A quantitative measure of EOL concerns in HD would enable a better understanding of how these concerns impact health-related quality of life. Therefore, we developed new measures of EOL for use in HD.
Methods
An EOL item pool of 45 items was field tested in 507 individuals with prodromal or manifest HD. Exploratory and confirmatory factor analyses (EFA and CFA, respectively) were conducted to establish unidimensional item pools. Item response theory (IRT) and differential item functioning analyses were applied to the identified unidimensional item pools to select the final items.
Results
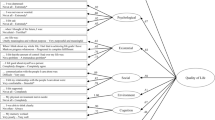
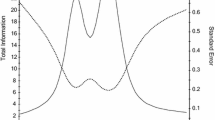
EFA and CFA supported two separate unidimensional sets of items: Concern with Death and Dying (16 items), and Meaning and Purpose (14 items). IRT and DIF supported the retention of 12 Concern with Death and Dying items and 4 Meaning and Purpose items. IRT data supported the development of both a computer adaptive test (CAT) and a 6-item, static short form for Concern with Death and Dying.
Conclusion
The HDQLIFE Concern with Death and Dying CAT and corresponding 6-item short form, and the 4-item calibrated HDQLIFE Meaning and Purpose scale demonstrate excellent psychometric properties. These new measures have the potential to provide clinically meaningful information about end-of-life preferences and concerns to clinicians and researchers working with individuals with HD. In addition, these measures may also be relevant and useful for other terminal conditions.




Similar content being viewed by others
References
Ross, C. A., Margolis, R. L., Rosenblatt, A., Ranen, N. G., Becher, M. W., & Aylward, E. (1997). Huntington disease and the related disorder, dentatorubral-pallidoluysian atrophy (DRPLA). Medicine (Baltimore), 76(5), 305–338.
Paulsen, J. S. (2010). Early detection of huntington disease. Future Neurology, 5(1), 85–104.
Carlozzi, N. E., & Tulsky, D. S. (2013). Identification of health-related quality of life (HRQOL) issues relevant to individuals with Huntington disease. Journal of Health Psychology, 18(2), 212–225.
Booij, S. J., Tibben, A., Engberts, D. P., Marinus, J., & Roos, R. A. C. (2014). Thinking about the end of life: A common issue for patients with Huntington’s disease. Journal of Neurology, 261(11), 2184–2191.
Teno, J. M., Byock, I., & Field, M. J. (1999). Research agenda for developing measures to examine quality of care and quality of life of patients diagnosed with life-limiting illness. Journal of Pain and Symptom Management, 17(2), 75–82.
Steinhauser, K. E., Clipp, E. C., & Tulsky, J. A. (2002). Evolution in measuring the quality of dying. Journal of Palliative Medicine, 5(3), 407–414.
Mularski, R. A., Dy, S. M., Shugarman, L. R., Wilkinson, A. M., Lynn, J., Shekelle, P. G., et al. (2007). A systematic review of measures of end-of-life care and its outcomes. Health Services Research, 42(5), 1848–1870.
van Soest-Poortvliet, M. C., van der Steen, J. T., Zimmerman, S., Cohen, L. W., Reed, D., Achterberg, W. P., et al. (2013). Selecting the best instruments to measure quality of end-of-life care and quality of dying in long term care. Journal of the American Medical Directors Association, 14(3), 179–186.
Albers, G., Echteld, M. A., de Vet, H. C., Onwuteaka-Philipsen, B. D., van der Linden, M. H., & Deliens, L. (2010). Content and spiritual items of quality-of-life instruments appropriate for use in palliative care: A review. Journal of Pain and Symptom Management, 40(2), 290–300.
Klager, J., Duckett, A., Sandler, S., & Moskowitz, C. (2008). Huntington’s disease: A caring approach to the end of life. Care Management Journal, 9(2), 75–81.
Huntington Society of Canada. (2010). Factsheets for healthcare professionals: Palliative care for individuals in late-stage Huntington disease. http://www.huntingtonsociety.ca/hd-fact-sheets-articles/hd-fact-sheets-in-black-white/Palliative_care_Health_Prof%20(6).pdf
Downing, N. R., Williams, J. K., & Paulsen, J. S. (2010). Couples’ attributions for work function changes in prodromal Huntington disease. Journal of Genetic Counseling, 19(4), 343–352.
McMillan, S. C., & Weitzner, M. (1998). Quality of life in cancer patients: Use of a revised Hospice Index. Cancer Practice, 6(5), 282–288.
Krause, S., Rydall, A., Hales, S., Rodin, G., & Lo, C. (2015). Initial validation of the death and dying distress scale for the assessment of death anxiety in patients with advanced cancer. Journal of Pain and Symptom Management, 49(1), 126–134.
Lo, C., Hales, S., Zimmermann, C., Gagliese, L., Rydall, A., & Rodin, G. (2011). Measuring death-related anxiety in advanced cancer: Preliminary psychometrics of the death and dying distress scale. Journal of Pediatric Hematology/Oncology, 33(Suppl 2), S140–S145.
Cohen, S. R., Mount, B. M., Bruera, E., Provost, M., Rowe, J., & Tong, K. (1997). Validity of the McGill quality of life questionnaire in the palliative care setting: A multi-centre Canadian study demonstrating the importance of the existential domain. Palliative Medicine, 11(1), 3–20.
Cohen, S. R., Mount, B. M., Strobel, M. G., & Bui, F. (1995). The mcgill quality of life questionnaire: A measure of quality of life appropriate for people with advanced disease. A preliminary study of validity and acceptability. Palliative Medicine, 9(3), 207–219.
Lo, C., Burman, D., Swami, N., Gagliese, L., Rodin, G., & Zimmermann, C. (2011). Validation of the QUAL-EC for assessing quality of life in patients with advanced cancer. European Journal of Cancer, 47(4), 554–560.
Heyland, D. K., Jiang, X., Day, A. G., Cohen, S. R., & Canadian Researchers at the End of Life Network. (2013). The development and validation of a shorter version of the Canadian Health Care Evaluation Project Questionnaire (CANHELP Lite): A novel tool to measure patient and family satisfaction with end-of-life care. Journal of Pain and Symptom Management, 46(2), 289–297.
McCaffrey, N., Skuza, P., Breaden, K., Eckermann, S., Hardy, J., Oaten, S., et al. (2014). Preliminary development and validation of a new end-of-life patient-reported outcome measure assessing the ability of patients to finalise their affairs at the end of life. PLoS One, 9(4), e94316.
Byock, I. R., & Merriman, M. P. (1998). Measuring quality of life for patients with terminal illness: The Missoula-VITAS quality of life index. Palliative Medicine, 12(4), 231–244.
Rudilla, D., Oliver, A., Galiana, L., & Barreto, P. (2016). A new measure of home care patients’ dignity at the end of life: The palliative patients’ dignity scale (PPDS). Palliative and Support Care, 14(2), 99–108.
Buzgova, R., Kozakova, R., Sikorova, L., Zelenikova, R., & Jarosova, D. (2016). Development and psychometric evaluation of patient needs assessment in palliative care (PNAP) instrument. Palliative and Support Care, 14(2), 129–137.
Lawton, M. P., Moss, M., Hoffman, C., Kleban, M. H., Ruckdeschel, K., & Winter, L. (2001). Valuation of life: A concept and a scale. Journal of Aging and Health, 13(1), 3–31.
Steinhauser, K. E., Bosworth, H. B., Clipp, E. C., McNeilly, M., Christakis, N. A., Parker, J., & Tulsky, J. A. (2002). Initial assessment of a new instrument to measure quality of life at the end of life. Journal of Palliative Medicine, 5(6), 829–841.
Heyland, D. K., Cook, D. J., Rocker, G. M., Dodek, P. M., Kutsogiannis, D. J., Skrobik, Y., et al. (2010). The development and validation of a novel questionnaire to measure patient and family satisfaction with end-of-life care: The Canadian Health Care Evaluation Project (CANHELP) questionnaire. Palliative Medicine, 24(7), 682–695.
Cella, D., Gershon, R., Lai, J. S., & Choi, S. (2007). The future of outcomes measurement: Item banking, tailored short-forms, and computerized adaptive assessment. Quality of Life Research, 16(Suppl 1), 133–141.
Carlozzi, N. E., Schilling, S. G., Lai, J.-S., Paulsen, J. S., Hahn, E. A., Perlmutter, J. S., Ross, C. A., Downing, N. R., Kratz, A. L., McCormack, M. K., Nance, M. A., Quaid, K. A., Stout, J., Gershon, R. C., Ready, R., Miner, J. A., Barton, S. K., Perlman, S. L., Rao, S. M., Frank, S., Shoulson, I., Marin, H., Geschwind, M. D., Dayalu, P., Foroud, T., Goodnight, S. M., & Cella, D. (Under Review). HDQLIFE: Development and assessment of health-related quality of life in Huntington disease (HD). Quality of Life Research.
Novack, T. (2000). The orientation log. Retrieved March 3, 2015, from http://www.tbims.org/combi/olog.
Hanauer, D. A., Mei, Q., Law, J., Khanna, R., & Zheng, K. (2015). Supporting information retrieval from electronic health records: A report of University of Michigan’s nine-year experience in developing and using the electronic medical record search engine (EMERSE). Journal of Biomedical Informatics, 55, 290–300.
Paulsen, J. S., Hayden, M., Stout, J. C., Langbehn, D. R., Aylward, E., Ross, C. A., et al. (2006). Preparing for preventive clinical trials: The predict-HD study. Archives of Neurology, 63(6), 883–890.
Paulsen, J. S., Langbehn, D. R., Stout, J. C., Aylward, E., Ross, C. A., Nance, M., et al. (2008). Detection of Huntington’s disease decades before diagnosis: The Predict-HD study. Journal of Neurology, Neurosurgery and Psychiatry, 79(8), 874–880.
Paulsen, J. S., Long, J. D., Johnson, H. J., Aylward, E. H., Ross, C. A., Williams, J. K., et al. (2014). Clinical and biomarker changes in premanifest Huntington disease show trial feasibility: A decade of the PREDICT-HD study. Front Aging Neuroscience, 6, 78.
Cella, D., Nowinski, C., Peterman, A., Victorson, D., Miller, D., Lai, J.-S., & Moy, C. (2011). The neurology quality of life measurement (Neuro-QOL) initiative. Archives of Physical Medicine and Rehabilitation, Supplement, 92(Suppl 1), S28–S36.
(2013). Neuro-QoL Final report. www.neuroqol.org/Resources/Resources%20documents/NeuroQOL-Final%20report-2013.pdf.
PROMIS® Instrument Development and Psychometric Evaluation Scientific Standards. http://www.nihpromis.org/Documents/PROMIS_Standards_050212.pdf.
Shoulson, I., & Fahn, S. (1979). Huntington disease—clinical care and evaluation. Neurology, 29(1), 1–3.
Huntington Study Group. (1996). Unified Huntington’s disease rating scale: Reliability and consistency. Movement Disorders, 11(2), 136–142.
Muthen, B. (1978). Contributions to factor-analysis of dichotomous variables. Psychometrika, 43(4), 551–560.
Muthen, B., Du Toit, S. H. C., & Spisic, D. (1997). Robust inference using weighted least squares and quadratic estimating equations in latent variable modeling with categorical and continuous outcomes. https://www.statmodel.com/download/Article_075.pdf.
Kline, R. B. (2005). Principles and practice of structural equation modeling (2nd ed.). New York: Guilford Press.
Bentler, P. M. (1990). Comparative fit indexes in structural models. Psychological Bulletin, 107(2), 238–246.
Hu, L. T., & Bentler, P. M. (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6(1), 1–55.
Hatcher, L. (1994). A step-by-step approach to using SAS for factor analysis and structural equation modeling. Cary: SAS Institute Inc.
McDonald, R. P. (1999). Test theory: A unified treatment. Mahwah: Lawrence Erlbaum Associates Inc.
Reise, S. P., Morizot, J., & Hays, R. D. (2007). The role of the bifactor model in resolving dimensionality issues in health outcomes measures. Quality of Life Research, 16(Suppl 1), 19–31.
Cook, K. F., Kallen, M. A., & Amtmann, D. (2009). Having a fit: Impact of number of items and distribution of data on traditional criteria for assessing IRT’s unidimensionality assumption. Quality of Life Research, 18(4), 447–460.
Muthén, L. K., & Muthén, B. O. (2011). Mplus user’s guide. Los Angeles: Muthén & Muthén.
Samejima, F., van der Liden, W. J., & Hambleton, R. (1996). The graded response model. In W. J. van der Liden (Ed.), Handbook of modern item response theory (pp. 85–100). New York, NY: Springer.
Cai, L., Thissen, D., & du Toit, S. H. C. (2011). IRTPRO for windows [Computer software]. Lincolnwood: Scientific Software International.
Crane, P. K., Gibbons, L. E., Jolley, L., & van Belle, G. (2006). Differential item functioning analysis with ordinal logistic regression techniques. DIFdetect and difwithpar. Medical Care, 44(11 Suppl 3), S115–S123.
R Core Team. (2014). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Choi, S. W. (2009). Firestar: Computerized adaptive testing simulation program for polytomous item response theory models. Applied Psychological Measurement, 33(8), 644–645.
Samejima, F. (1969). Estimation of latent ability using a response pattern of graded scores (Psychometric Monograph No. 17). Richmond, VA: Psychometric Society.
Bryant, F. B., & Yarnold, P. R. (1995). Principal components analysis and exploratory and confirmatory factor analysis. In L. G. Grimm & R. R. Yarnold (Eds.), Reading and understanding multivariate statistics (pp. 99–136). Washington, DC: American Psychological Association.
Everitt, B. S. (1975). Multivariate analysis: The need for data, and other problems. British Journal of Psychiatry, 126, 237–240.
Gorsuch, R. L. (1983). Factor analysis. Hillsdale: Lawrence Erlbaum Associates.
Clauser, B. E., & Hambleton, R. K. (1994). Review of differential item functioning, P. W. Holland, H. Wainer. Journal of Educational Measurement, 31(1), 88–92.
Kirwin, J. L., & Edwards, R. A. (2013). Helping patients articulate end-of-life wishes: A target for interprofessional participation. Annals of Palliative Medicine, 2(2), 95–97.
Booij, S. J., Engberts, D. P., Rodig, V., Tibben, A., & Roos, R. A. (2013). A plea for end-of-life discussions with patients suffering from Huntington’s disease: The role of the physician. Journal of Medical Ethics, 39(10), 621–624.
Booij, S. J., Tibben, A., Engberts, D. P., & Roos, R. A. (2014). Perhaps the subject of the questionnaire was too sensitive: Do we expect too much too soon? Wishes for the end of life in Huntington’s disease—The perspective of European physicians. J Huntingtons Disease, 3(3), 229–232.
Dellefield, M. E., & Ferrini, R. (2011). Promoting excellence in end-of-life care: Lessons learned from a cohort of nursing home residents with advanced Huntington disease. Journal of Neuroscience Nursing, 43(4), 186–192.
Williams, J. K., Erwin, C., Juhl, A. R., Mengeling, M., Bombard, Y., Hayden, M. R., et al. (2010). In their own words: Reports of stigma and genetic discrimination by people at risk for Huntington disease in the International RESPOND-HD study. American Journal of Medical Genetics B Neuropsychiatric Genetics, 153B(6), 1150–1159.
Boersma, I., Miyasaki, J., Kutner, J., & Kluger, B. (2014). Palliative care and neurology: Time for a paradigm shift. Neurology, 83(6), 561–567.
Tibben, A. (2007). Predictive testing for Huntington’s disease. Brain Research Bulletin, 72(2–3), 165–171.
Duff, K., Paulsen, J. S., Beglinger, L. J., Langbehn, D. R., Wang, C., Stout, J. C., et al. (2010). “Frontal” behaviors before the diagnosis of Huntington’s disease and their relationship to markers of disease progression: Evidence of early lack of awareness. Journal of Neuropsychiatry and Clinical Neurosciences, 22(2), 196–207.
Acknowledgments
Work on this manuscript was supported by the National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke (R01NS077946), and the National Center for Advancing Translational Sciences (UL1TR000433). In addition, a portion of this study sample was collected in conjunction with the Predict-HD study. The Predict-HD was supported by the NIH, National Institute of Neurological Disorders and Stroke (R01NS040068), the NIH, Center for Inherited Disease Research (provided supported for sample phenotyping), and the CHDI Foundation (award to the University of Iowa). We thank the University of Iowa, the Investigators and Coordinators of this study, the study participants, the National Research Roster for Huntington Disease Patients and Families, the Huntington Study Group, and the Huntington’s Disease Society of America. We acknowledge the assistance of Jeffrey D. Long, Hans J. Johnson, Jeremy H. Bockholt, Roland Zschiegner, and Jane S. Paulsen. We also acknowledge Roger Albin, Kelvin Chou, and Henry Paulsen for the assistance with participant recruitment. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. HDQLIFE Site Investigators and Coordinators are Noelle Carlozzi, Praveen Dayalu, Stephen Schilling, Amy Austin, Matthew Canter, Siera Goodnight, Jennifer Miner, Nicholas Migliore (University of Michigan, Ann Arbor, MI); Jane Paulsen, Nancy Downing, Isabella DeSoriano, Courtney Shadrick, Amanda Miller (University of Iowa, Iowa City, IA); Kimberly Quaid, Melissa Wesson (Indiana University, Indianapolis, IN); Christopher Ross, Gregory Churchill, Mary Jane Ong (Johns Hopkins University, Baltimore, MD); Susan Perlman, Brian Clemente, Aaron Fisher, Gloria, Obialisi, Michael Rosco (University of California Los Angeles, Los Angeles, CA); Michael McCormack, Humberto Marin, Allison Dicke (Rutgers University, Piscataway, NJ); Joel Perlmutter, Stacey Barton, Shineeka Smith (Washington University, St. Louis, MO); Martha Nance, Pat Ede (Struthers Parkinson’s Center); Stephen Rao, Anwar Ahmed, Michael Lengen, Lyla Mourany, Christine Reece, (Cleveland Clinic Foundation, Cleveland, OH); Michael Geschwind, Joseph Winer (University of California—San Francisco, San Francisco, CA), David Cella, Richard Gershon, Elizabeth Hahn, Jin-Shei Lai (Northwestern University, Chicago, IL).
Funding
Work on this manuscript was supported by the National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke (R01NS077946), and the National Center for Advancing Translational Sciences (UL1TR000433). In addition, a portion of this study sample was collected in conjunction with the Predict-HD study. The Predict-HD was supported by the NIH, National Institute of Neurological Disorders and Stroke (R01NS040068), the NIH Center for Inherited Disease Research (provided supported for sample phenotyping), and the CHDI Foundation (award to the University of Iowa).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Carlozzi, N.E. currently has research grants from the NIH; she is also supported by grant funding from the NIH, NIDILRR, and CHDI; she declares no conflicts of interest. Downing, N.R. declares no conflicts of interest. McCormack, M.K. currently has grants from the NJ Department of Health; he declare no conflicts of interest. Schilling, S.G. has a research grant from NSF. He also is supported by grant funding from NIH. He declares no conflicts of interest. Perlmutter, J.S. currently has funding from the NIH, HDSA, CHDI, and APDA. He has received honoraria from the University of Rochester, American Academy of Neurology, Movement Disorders Society, Toronto Western Hospital, St Lukes Hospital in St Louis, Emory U, Penn State, Alberta innovates, Indiana Neurological Society, Parkinson Disease Foundation, Columbia University, St. Louis University, Harvard University, and the University of Michigan. Hahn, E.A. currently has research grants from the NIH; she is also supported by grant funding from the NIH and PCORI, and by research contracts from Merck and EMMES; she declares no conflicts of interest. Lai J.-S. currently has research grants from the NIH; she declares no conflicts of interest. Frank, S. receives salary support from the Huntington Study Group for a study sponsored by Auspex Pharmaceuticals. There is no conflict of interest. Quaid, K.A. has research funding from NIH, NIA, NCRR, NINDS and CHDI. She also has funding from HDSA. She has no conflicts of interest to declare. Paulsen, J.S. currently has research grants from the NIH; she is also supported by grant funding from NIH, NINDS, and CHDI; she declares no conflicts of interest. Cella, D. receives grant funding from the National Institutes of Health and reports that he has no conflicts of interest. Goodnight, S.M. is supported by grant funding from the NIH and the Craig H. Neilsen Foundation; she declares no conflicts of interest. Miner, J.A. is supported by research grants from the NIH; she declares no conflict of interest. Nance, M.A. declares no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Carlozzi, N.E., Downing, N.R., McCormack, M.K. et al. New measures to capture end of life concerns in Huntington disease: Meaning and Purpose and Concern with Death and Dying from HDQLIFE (a patient-reported outcomes measurement system). Qual Life Res 25, 2403–2415 (2016). https://doi.org/10.1007/s11136-016-1354-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-016-1354-y