Abstract
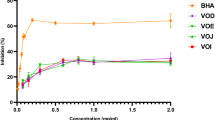
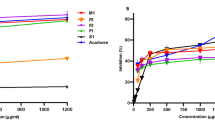
The beneficial health effects of apple consumption are well known, however, little is known about the potential of its phenolic fractions to inhibit α-glucosidases and thereafter to treat diseases related to the carbohydrate metabolism, such as postprandial hyperglycemia and diabetes. In the present study, the α-glucosidase inhibition and antioxidant activity of different phenolic fractions of apple were evaluated using the 2,2-diphenyl-1-picrylhydrazyl and hydroxyl radical scavenging activity. Moreover, the phenolic fractions were chemically characterized by LC-MS in order to identify the compounds responsible for the biological properties. The purified extract (not fractionated) had the highest α-glucosidase and hydroxyl radical inhibitions. The purified extract and fractions III and IV were more active against the enzyme activity than the positive control acarbose, the drug used by diabetic patients to treat postprandial hyperglycaemia. Our results show that apple phenolic extracts strongly inhibit α-glucosidase acitivity, validating their potential to be used in the management of type 2 diabetes.
Similar content being viewed by others
References
Bondonno NP, Bondonno CP, Ward NC, Hodgson JM, Croft KD (2017) The cardiovascular health benefits of apples: whole fruit vs. isolated compounds. Trends Food Sci Technol 69:243–256. https://doi.org/10.1016/j.tifs.2017.04.012
Zimmet P, Alberti KG, Magliano DJ, Bennett PH (2016) Diabetes mellitus statistics on prevalence and mortality: facts and fallacies. Nat Rev Endocrinol 12:616–622. https://doi.org/10.1038/nrendo.2016.105
World Health Organization (2018) Diabetes mellitus fact sheet N° 138. WHO media centre. http://www.who.int/mediacentre/factsheets/fs138/en/. Accessed 15 october 2018
World Health Organization. Global report on diabetes (2016) WHO media centre. http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf. Accessed 22 january 2019
Kwon YI, Apostolidis E, Kim YC, Shetty K (2007) Health benefits of traditional corn, beans, and pumpkin: In vitro studies for hyperglycemia and hypertension management. J Med Food 10:266–275. https://doi.org/10.1089/jmf.2006.234
Fujisawa T, Ikegami H, Inoue K, Kawabata Y, Ogihara T (2005) Effect of two α-glucosidase inhibitors, voglibose and acarbose, on postprandial hyperglycemia correlates with subjective abdominal symptoms. Metabolism 54:387–390. https://doi.org/10.1016/j.metabol.2004.10.004
Kim JS, Kwon CS, Son KH (2000) Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci Biotechnol Biochem 64:2458–2461. https://doi.org/10.1271/bbb.64.2458
Zaklos-Szyda M, Majewska I, Redzynia M, Koziolkiewic M (2015) Antidiabetic effect of polyphenolic extracts from selected edible plants as α-amylase, α-glucosidase and PTP1B inhibitors, and β pancreatic cells cytoprotective agents-a comparative study. Curr Top Med Chem 15:2431–2444. https://doi.org/10.2174/1568026615666150619143051
Muraki I, Imamura F, Manson JE, Hu FB, Willett WC, van Dam RM, Sun Q (2013) Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ 347:f5001. https://doi.org/10.1136/bmj.f5001
Guo X, Yang B, Tang J, Jiang JJ, Li D (2017) Apple and pear consumption and type 2 diabetes mellitus risk: a meta-analysis of prospective cohort studies. Food Funct 8:927–934. https://doi.org/10.1039/c6fo01378c
Boyer J, Liu RH (2004) Apple phytochemicals and their health benefits. Nutrition 3:5. https://doi.org/10.1186/1475-2891-3-5
Francini A, Sebastiani L (2013) Phenolic compounds in apple (Malus x domestica Borkh.): compounds characterization and stability during postharvest and after processing. Antioxidants 2:181–193. https://doi.org/10.3390/antiox2030181
Adyanthaya I, Kwon YI, Apostolidis E, Shetty K (2010) Health benefits of apple phenolics from postharvest stages for potential type 2 diabetes management using in vitro models. J Food Biochem 34:31–49. https://doi.org/10.1111/J.1745-4514.2009.00257.X
Cornille A, Feurtey A, Gélin U, Ropars J, Misvanderbrugge K, Gladieux P, Giraud T (2015) Anthropogenic and natural drivers of gene flow in a temperate wild fruit tree: a basis for conservation and breeding programs in apples. Evol Appl 8:373–384. https://doi.org/10.1111/eva.12250
Oszmianski J, Ramos T, Bourzeix M (1988) Fractionation of phenolic compounds in red wine. Am J Enol Viticult 39:259–262
Swain T, Hillis WE (1959) The phenolic constituents of Prunus domestica. I.—the quantitative analysis of phenolic constituents. J Sci Food Agric 10:63–68. https://doi.org/10.1002/jsfa.2740100110
Vinholes J, Lemos G, Lia Barbieri R, Franzon RC, Vizzotto M (2017) In vitro assessment of the antihyperglycemic and antioxidant properties of araçá, butiá and Pitanga. Food Biosci 19:92–100. https://doi.org/10.1016/j.fbio.2017.06.005
Tresserra-Rimbau A et al (2016) Intake of total polyphenols and some classes of polyphenols is inversely associated with diabetes in elderly people at high cardiovascular disease risk. J Nutr 146:767–777. https://doi.org/10.3945/jn.115.223610
Rasines-Perea Z, Teissedre PL (2017) Grape polyphenols’ effects in human cardiovascular diseases and diabetes. Molecules 22:68. https://doi.org/10.3390/molecules22010068
Renda G, Özel A, Barut B, Korkmaz B, Šoral M, Kandemir Ü, Liptaj T (2017) Bioassay guided isolation of active compounds from Alchemilla barbatiflora Juz. Rec Nat Prod 12:76–85. https://doi.org/10.25135/rnp.07.17.07.117
Rahman MJ, de Camargo AC, Shahidi F (2017) Phenolic and polyphenolic profiles of chia seeds and their in vitro biological activities. J Funct Foods 35:622–634. https://doi.org/10.1016/j.jff.2017.06.044
Tamura T, Ozawa M, Kobayashi S, Watanabe H, Arai S, Mura K (2015) Inhibitory effect of oligomeric polyphenols from peanut-skin on sugar digestion enzymes and glucose transport. Food Sci Technol Res 21:111–115. https://doi.org/10.3136/fstr.21.111
Wei M, Chai W-M, Yang Q, Wang R, Peng Y (2017) Novel insights into the inhibitory effect and mechanism of proanthocyanidins from Pyracantha fortuneana fruit on α-glucosidase. J Food Sci 82:2260–2268. https://doi.org/10.1111/1750-3841.13816
de Camargo AC, Regitano-d’Arce MAB, Biasoto ACT, Shahidi F (2016) Enzyme-assisted extraction of phenolics from winemaking by-products: antioxidant potential and inhibition of alpha-glucosidase and lipase activities. Food Chem 212:395–402. https://doi.org/10.1016/j.foodchem.2016.05.047
Kalita D, Holm DG, LaBarbera DV, Petrash JM, Jayanty SS (2018) Inhibition of α-glucosidase, α-amylase, and aldose reductase by potato polyphenolic compounds. PLoS One 13:e0191025. https://doi.org/10.1371/journal.pone.0191025
Xu D, Wang Q, Zhang W, Hu B, Zhou L, Zeng X, Sun Y (2015) Inhibitory activities of caffeoylquinic acid derivatives from Ilex kudingcha C.J. Tseng on α-glucosidase from Saccharomyces cerevisiae. J Agric Food Chem 63:3694–3703. https://doi.org/10.1021/acs.jafc.5b00420
Schmidt JS, Lauridsen MB, Dragsted LO, Nielsen J, Staerk D (2012) Development of a bioassay-coupled HPLC-SPE-ttNMR platform for identification of α-glucosidase inhibitors in apple peel (Malus domestica Borkh.). Food Chem 135:1692–1699. https://doi.org/10.1016/j.foodchem.2012.05.075
Meng Y, Su A, Yuan S, Zhao H, Tan S, Hu C, Deng H, Guo Y (2016) Evaluation of total flavonoids, myricetin, and quercetin from Hovenia dulcis Thunb. as inhibitors of α-amylase and α-glucosidase. Plant Foods Hum Nutr 71:444–449. https://doi.org/10.1007/s11130-016-0581-2
Proença C, Freitas M, Ribeiro D, Oliveira EFT, Sousa JLC, Tomé SM, Ramos MJ, Silva AMS, Fernandes PA, Fernandes E (2017) α-Glucosidase inhibition by flavonoids: an in vitro and in silico structure–activity relationship study. J Enzyme Inhib Med Chem 32:1216–1228. https://doi.org/10.1080/14756366.2017.1368503
Han L, Fang C, Zhu R, Peng Q, Li D, Wang M (2017) Inhibitory effect of phloretin on α-glucosidase: kinetics, interaction mechanism and molecular docking. Int J Biol Macromol 95:520–527. https://doi.org/10.1016/j.ijbiomac.2016.11.089
Johnston KL, Clifford MN, Morgan LM (2002) Possible role for apple juice phenolic compounds in the acute modification of glucose tolerance and gastrointestinal hormone secretion in humans. J Sci Food Agric 82:1800–1805. https://doi.org/10.1002/jsfa.1264
Marks SC, Mullen W, Borges G, Crozier A (2009) Absorption, metabolism, and excretion of cider dihydrochalcones in healthy humans and subjects with an ileostomy. J Agric Food Chem 57:2009–2015. https://doi.org/10.1021/jf802757x
Alberti A, dos Santos TPM, Zielinski AAF, dos Santos CME, Braga CM, Demiate IM, Nogueira A (2016) Impact on chemical profile in apple juice and cider made from unripe, ripe and senescent dessert varieties. LWT - Food Sci Technol 65:436–443. https://doi.org/10.1016/j.lwt.2015.08.045
Rao KS, Chaudhury PK, Pradhan A (2010) Evaluation of anti-oxidant activities and total phenolic content of Chromolaena odorata. Food Chem Toxicol 48:729–732. https://doi.org/10.1016/j.fct.2009.12.005
Saravanan S, Parimelazhagan T (2014) In vitro antioxidant, antimicrobial and anti-diabetic properties of polyphenols of Passiflora ligularis Juss. fruit pulp. Food Sci Hum Wellness 3:56–64. https://doi.org/10.1016/j.fshw.2014.05.001
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Oliveira Raphaelli, C., dos Santos Pereira, E., Camargo, T.M. et al. Apple Phenolic Extracts Strongly Inhibit α-Glucosidase Activity. Plant Foods Hum Nutr 74, 430–435 (2019). https://doi.org/10.1007/s11130-019-00757-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-019-00757-3