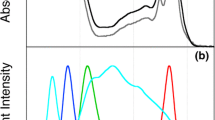
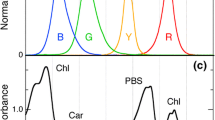
Abstract
Fucoxanthin-chlorophyll (Chl) a/c–binding proteins (FCPs) are light-harvesting pigment–protein complexes found in diatoms and brown algae. Due to the characteristic pigments, such as fucoxanthin and Chl c, FCPs can capture light energy in blue-to green regions. A pennate diatom Phaeodactylum tricornutum synthesizes a red-shifted form of FCP under weak or red light, extending a light-absorption ability to longer wavelengths. In the present study, we examined changes in light-harvesting and energy-transfer processes of P. tricornutum cells grown under white- and single-colored light-emitting diodes (LEDs). The red-shifted FCP appears in the cells grown under the green, yellow, and red LEDs, and exhibited a fluorescence peak around 714 nm. Additional energy-transfer pathways are established in the red-shifted FCP; two forms (F713 and F718) of low-energy Chl a work as energy traps at 77 K. Averaged fluorescence lifetimes are prolonged in the cells grown under the yellow and red LEDs, whereas they are shortened in the blue-LED-grown cells. Based on these results, we discussed the light-adaptation machinery of P. tricornutum cells involved in the red-shifted FCP.






Similar content being viewed by others
Abbreviations
- AFDA:
-
Absolute fluorescence decay-associated
- Car:
-
Carotenoid
- Chl:
-
Chlorophyll
- FCP:
-
Fucoxanthin Chl a/c-binding protein
- FDA:
-
Fluorescence decay-associated
- LED:
-
Light-emitting diode
- LHC:
-
Light-harvesting complex
- PS:
-
Photosystem
- RC:
-
Reaction center
- TRF:
-
Time-resolved fluorescence
References
Akimoto S, Yokono M, Hamada F, Teshigahara A, Aikawa S, Kondo A (2012) Adaptation of light-harvesting systems of Arthrospira platensis to light conditions, probed by time-resolved fluorescence spectroscopy. Biochim Biophys Acta 1817:1483–1489
Akimoto S, Yokono M, Yokono E, Aikawa S, Kondo A (2014) Short-term light adaptation of a cyanobacterium, Synechocystis sp. PCC 6803, probed by time-resolved fluorescence spectroscopy. Plant Physiol Biochem 81:149–154
Akimoto S, Shinoda T, Chen M, Allakhverdiev SI, Tomo T (2015) Energy transfer in the chlorophyll f-containing cyanobacterium, Halomicronema hongdechloris, analyzed by time-resolved fluorescence spectroscopies. Photosynth Res 125:115–122
Blankenship RE (2014) Molecular mechanisms of photosynthesis, 2nd edn. Wiley-Blackwell, Oxford
Brettel K, Leibl W (2001) Electron transfer in photosystem I. Biochim Biophys Acta 1507:100–114
Brown JS (1967) Fluorometric evidence for the participation of chlorophyll a-695 in System 2 of photosynthesis. Biochim Biophys Acta 143:391–398
Busch A, Hippler M (2011) The structure and function of eukaryotic photosystem I. Biochim Biophys Acta 1807:864–877
Chen M, Schliep M, Willows RD, Cai Z-L, Neilan BA, Scheer H (2010) A red-shifted chlorophyll. Science 329:1318–1319
Chen M, Li Y, Birch D, Willows RD (2012) A cyanobacterium that contains chlorophyll f–a red-absorbing photopigment. FEBS Lett 586:3249–3254
Diner BA, Rappaport F (2002) Structure, dynamics, and energetics of the primary photochemistry of photosystem II of oxygenic photosynthesis. Annu Rev Plant Biol 53:551–580
Field CB, Behrenfeld MJ, Randerson JT, Falkowski P (1998) Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281:237–240
Herbstová M, Bína D, Koník P, Gardian Z, Vácha F, Litvín R (2015) Molecular basis of chromatic adaptation in pennate diatom Phaeodactylum tricornutum. Biochim Biophys Acta 1847:534–543
Herbstová M, Bína D, Kaňa R, Vácha F, Litvín R (2017) Red-light phenotype in a marine diatom involves a specialized oligomeric red-shifted antenna and altered cell morphology. Sci Rep 7:11976
Ishihara T, Ifuku K, Yamashita E, Fukunaga Y, Nishino Y, Miyazawa A, Kashino Y, Inoue-Kashino N (2015) Utilization of light by fucoxanthin-chlorophyll-binding protein in a marine centric diatom, Chaetoceros gracilis. Photosynth Res 126:437–447
Kato K, Nagao R, Jiang T-Y, Ueno Y, Yokono M, Chan SK, Watanabe M, Ikeuchi M, Shen J-R, Akimoto S, Miyazaki N, Akita F (2019) Structure of a cyanobacterial photosystem I tetramer revealed by cryo-electron microscopy. Nat Commun 10:4929
Lavaud J, Lepetit B (2013) An expanation for the inter-species variability of the photoprotective non-photochemical chlorophyll fluorescence quenching in diatoms. Biochim Biophys Acta 1827:294–302
Mirkovic T, Ostroumov EE, Anna JM, van Grondelle R, Govindjee SGD (2017) Light absorption and energy transfer in the antenna complexes of photosynthetic organisms. Chem Rev 117:249–293
Nagao R, Ishii A, Tada O, Suzuki T, Dohmae N, Okumura A, Iwai M, Takahashi T, Kashino Y, Enami I (2007) Isolation and characterization of oxygen-evolving thylakoid membranes and Photosystem II particles from a marine diatom Chaetoceros gracilis. Biochim Biophys Acta 1767:1353–1362
Nagao R, Ueno Y, Yokono M, Shen J-R, Akimoto S (2018) Alterations of pigment composition and their interactions in response to different light conditions in the diatom Chaetoceros gracilis probed by time-resolved fluorescence spectroscopy. Biochim Biophys Acta 1859:524–530
Nagao R, Ueno Y, Yokono M, Shen JR, Akimoto S (2019) Effects of excess light energy on excitation-energy dynamics in a pennate diatom Phaeodactylum tricornutum. Photosynth Res 141:355–365
Neilson JAD, Durnford DG (2010) Structural and functional diversification of the light-harvesting complexes in photosynthetic eukaryotes. Photosynth Res 106:57–71
Nymark M, Volpe C, Hafskjold MCG, Kirst H, Serif M, Vadstein O, Bones AM, Melis A, Winge P (2019) Loss of ALBINO3b insertase results in truncated light-harvesting antenna in diatoms. Plant Physiol 181:1257–1276
Pagliano C, Saracco G, Barber J (2013) Structural, functional and auxiliary proteins of photosystem II. Photosynth Res 116:167–188
Shubin VV, Bezsmertnaya IN, Karapetyan NV (1995) Efficient energy transfer from the long-wavelength antenna chlorophylls to P700 in photosystem I complexes from Spirulina platensis. J Photochem Photobiol B 30:153–160
Tomo T, Shinoda T, Chen M, Allakhverdiev SI, Akimoto S (2014) Energy transfer processes in chlorophyll f-containing cyanobacteria using time-resolved fluorescence spectroscopy on intact cells. Biochim Biophys Acta 1837:1484–1489
Ueno Y, Shimakawa G, Miyake C, Akimoto S (2018) Light-harvesting strategy during CO2-dependent photosynthesis in the green alga Chlamydomonas reinhardtii. J Phys Chem Lett 9:1028–1033
Ueno Y, Nagao R, Shen J-R, Akimoto S (2019) Spectral properties and excitation relaxation of novel fucoxanthin chlorophyll a/c-binding protein complexes. J Phys Chem Lett 10:5148–5152
Yokono M, Akimoto S, Koyama K, Tsuchiya T, Mimuro M (2008) Energy transfer processes in Gloeobacter violaceus PCC 7421 that possesses phycobilisomes with a unique morphology. Biochim Biophys Acta 1777:55–65
Yokono M, Nagao R, Tomo T, Akimoto S (2015) Regulation of excitation energy transfer in diatom PSII dimer: how does it change the destination of excitation energy? Biochim Biophys Acta 1847:1274–1282
Yokono M, Takabayashi A, Kishimoto J, Fujita T, Iwai M, Murakami A, Akimoto S, Tanaka A (2019) The PSI-PSII megacomplex in green plants. Plant Cell Physiol 60:1098–1108
Acknowledgements
This work was supported by the Grants-in-Aid for Scientific Research from Japan Society for the Promotion of Science KAKENHI JP18J10095 (to Y.U.), JP17H06433 (to J.-R.S.), JP17K07442 and JP19H04726 (to R.N.), and JP16H06553 (to S.A.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oka, K., Ueno, Y., Yokono, M. et al. Adaptation of light-harvesting and energy-transfer processes of a diatom Phaeodactylum tricornutum to different light qualities. Photosynth Res 146, 227–234 (2020). https://doi.org/10.1007/s11120-020-00714-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-020-00714-1