Abstract
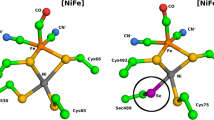
The results of homology modeling of HydSL, a NiFe-hydrogenase from purple sulfur bacterium Thiocapsa roseopersicina BBS, and deep-water bacterium Alteromonas macleodii deep ecotype are presented in this work. It is shown that the models have larger confidence level than earlier published ones; full-size models of these enzymes are presented for the first time. The C-end fragment of small subunit of T. roseopersicina hydrogenase is shown to have random orientation in relation to the main protein globule. The obtained models of this enzyme have a large number of ion pairs, as well as thermostable HydSL hydrogenase from Allochromatium vinosum, in contrast to thermostable HydSL hydrogenase from Alt. macleodii and thermolabile HydAB hydrogenase from Desulfovibrio vulgaris. The possible determinant of oxygen stability of studied hydrogenases could be the lack of several intramolecular tunnels. Hydrophobic and electrostatic surfaces were mapped in order to find out possible pathways of coupling hydrogenase to electron-transferring chains, as well as methods for construction of artificial photobiohydrogen-producing systems.






Similar content being viewed by others
References
Abdullatypov AV, Tsygankov AA (2013) Homology modeling of the spatial structure of HydSL hydrogenase from purple sulfur bacterium Thiocapsa roseopersicina BBS. Comput Res Model 5(4):737–747 (Russian)
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410. doi:10.1016/S0022-2836(05)80360-2
Berka K, Hanak O, Sehnal, D, Banas P, Navratilova V, Jaiswal D, Ionescu C-M, Svobodova Varekova R, Koča J, Otyepka M (2012) MOLEonline 2.0: interactive web-based analysis of biomacromolecular channels. Nucleic Acid Res. 40(W1): W222–W227. doi: 10.1093/nar/GKS363
Buhrke T, Lenz O, Krauss N, Friedrich B (2005) Oxygen tolerance of the H2-sensing [NiFe] hydrogenase from Ralstonia eutropha H16 is based on limited access of oxygen to the active site. J Biol Chem 280(25):23791–23796. doi:10.1074/jbc.M503260200
Chen VB, Arendall WB, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC (2010) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D 66:12–21. doi:10.1107/S0907444909042073
Duan Y, Wu C, Chowdhury S, Lee MC, Xiong G, Zhang W, Yang R, Cieplak P, Luo R, Lee T, Caldwell J, Wang J, Kollman P (2003) A point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculations. J Comput Chem 24(16):1999–2012. doi:10.1002/jcc.10349
Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG (1995) A smooth particle mesh Ewald method. J Chem Phys 103(19):8577–8593. doi:10.1063/1.470117
Frielingsdorf S, Fritsch J, Schmidt A, Hammer M, Löwenstein J, Siebert E, Pelmenschikov V, Jaenicke T, Kalms J, Rippers Y, Lendzian F, Zebger I, Teutloff C, Kaupp M, Bittl R, Hildebrandt P, Friedrich B, Lenz O, Scheerer P (2014) Reversible [4Fe-3S] cluster morphing in an O2-tolerant [NiFe] hydrogenase. Nat Chem Biol 10(5):378–385. doi:10.1038/nchembio.1500
Furnham N, Holliday GL, de Beer TA, Jacobsen JO, Pearson WR, Thornton JM (2014) The Catalytic Site Atlas 2.0: cataloging catalytic sites and residues identified in enzymes. Nucleic Acids Res 42(Database issue):D485–489. doi: 10.1093/nar/gkt1243
Gogotov IN, Zorin NA, Serebryakova LT, Kondratieva EN (1978) The properties of hydrogenase from Thiocapsa roseopersicina. Biochim Biophys Acta 523:335–343. doi:10.1016/0005-2744(78)90036-0
Higuchi Y, Yagi T, Yasuoka N (1997) Unusual ligand structure in Ni–Fe active center and an additional Mg site in hydrogenase revealed by high resolution X-ray structure analysis. Structure 5(12):1671–1680. doi:10.1016/S0969-2126(97)00313-4
Kelley LA, Sternberg MJE (2009) Protein structure prediction on the web: a case study using the Phyre server. Nat Protoc 4(3):363–371. doi:10.1038/nprot.2009.2
Klibanov AM, Kaplan NO, Karmen MD (1980) Thermal stabilities of membrane-bound, solubilized, and artificially immobilized hydrogenase from Chromatium vinosum. Arch Biochem Biophys 199(2):545–549. doi:10.1016/0003-9861(80)90312-4
Konagurthu AS, Whisstock JC, Stuckey PJ, Lesk AM (2006) MUSTANG: a multiple structural alignment algorithm. Proteins 64(3):559–574. doi:10.1002/prot.20921
Krieger E, Joo K, Lee J, Lee J, Raman S, Thompson J, Tyka M, Baker D, Karplus K (2009) Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: four approaches that performed well in CASP8. Proteins 77(Suppl 9):114–122. doi:10.1002/prot.22570
Krieger E, Dunbrack RL Jr, Hooft RW, Krieger B (2012) Assignment of protonation states in proteins and ligands: combining pKa prediction with hydrogen bonding network optimization. Methods Mol Biol 819:405–421. doi:10.1007/978-1-61779-465-0_25
Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157:105–132. doi:10.1016/0022-2836(82)90515-0
Laurinavichene TV, Rákhely G, Kovács KL, Tsygankov AA (2007) The effect of sulfur compounds on H2 evolution/consumption reactions, mediated by various hydrogenases, in the purple sulfur bacterium, Thiocapsa roseopersicina. Arch Microbiol 188(4):403–410. doi:10.1007/s00203-007-0260-7
Leroux F, Dementin S, Burlat B, Cournac L, Volbeda A, Champ S, Martin L, Guigliarelli B, Bertrand P, Fontecilla-Camps J, Rousset M, Leger C (2008) Experimental approaches to kinetics of gas diffusion in hydrogenase. Proc Natl Acad Sci USA 105(32):11188–11193. doi:10.1073/pnas.0803689105
Nicolet Y, Cavazza C, Fontecilla-Camps JC (2002) Fe-only hydrogenases: structure, function and evolution. J Inorg Biochem 91(1):1–8. doi:10.1016/S0162-0134(02)00392-6
Ogata H, Kellers P, Lubitz W (2010) The crystal structure of the [NiFe] hydrogenase from the photosynthetic bacterium Allochromatium vinosum: characterization of the oxidized enzyme (Ni-A state). J Mol Biol 402(2):428–444. doi:10.1016/j.jmb.2010.07.041
Palágyi-Mészáros LS, Maróti J, Latinovics D, Balogh T, Klement E, Medzihradszky KF, Rákhely G, Kovács KL (2009) Electron-transfer subunits of the NiFe hydrogenases in Thiocapsa roseopersicina BBS. FEBS J 276(1):164–174. doi:10.1111/j.1742-4658.2008.06770.x
Parkin A, Goldet G, Cavazza C, Fontecilla-Camps JC, Armstrong FA (2008) The difference a Se makes? Oxygen-tolerant hydrogen production by the [NiFeSe]-hydrogenase from Desulfomicrobium baculatum. J Am Chem Soc 130(40):13410–13416. doi:10.1021/ja803657d
Pieper U, Eswar N, Braberg H, Madhusudhan MS, Davis FP, Stuart AC, Mirkovic N, Rossi A, Marti-Renom MA, Fiser A, Webb B, Greenblatt D, Huang CC, Ferrin TE, Sali A (2011) MODBASE, a database of annotated comparative protein structure models and associated resources. Nucleic Acids Res 39:465–474. doi:10.1093/nar/gkq1091
Sali A, Blundell TL (1993) Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234(3):779–815. doi:10.1006/jmbi.1993.1626
Shen MY, Sali A (2006) Statistical potential for assessment and prediction of protein structures. Protein Sci 15(11):2507–2524. doi:10.1110/ps.062416606
Sherman MB, Orlova EV, Smirnova EA, Hovmöller S, Zorin NA (1991) Three-dimensional structure of the nickel-containing hydrogenase from Thiocapsa roseopersicina. J Bacteriol 173(8):2576–2580
Shomura Y, Hagiya K, Yoon KS, Nishihara H, Higuchi Y (2011) Crystallization and preliminary X-ray diffraction analysis of membrane-bound respiratory [NiFe] hydrogenase from Hydrogenovibrio marinus. Acta Crystallogr Sect F, Struct Biol Commun 67(7):827–829. doi:10.1107/S1744309111019804
Szilágyi A, Kovács KL, Rákhely G, Závodszky P (2002) Homology modeling reveals the structural background of the striking difference in thermal stability between two related [NiFe] hydrogenases. J Mol Model 8(2):58–64. doi:10.1007/s00894-001-0071-8
Thauer RK (1998) Biochemistry of methanogenesis: a tribute to Marjory Stephenson. Marjory Stephenson Prize Lecture. Microbiology 144:2377. doi:10.1099/00221287-144-9-2377
Theobald DL, Wuttke DS (2006) THESEUS: maximum likelihood superpositioning and analysis of macromolecular structures. Bioinformatics 22:2171–2172. doi:10.1093/bioinformatics/btl332
Tina KG, Bhadra R, Srinivasan N (2007) PIC: protein interactions calculator. Nucleic Acids Res 35 Web Server Issue: 473–476. doi: 10.1093/nar/gkm423
Tsygankov AA, Minakov EA, Zorin NA, Gosteva KS, Voronin OG, Karyakin AA (2007) Measuring the pH dependence of hydrogenase activities. Biochemistry (Moscow) 72(9):968–973. doi:10.1134/S0006297907090076
Vargas WA, Weyman PD, Tong Y, Smith HO, Xu Q (2011) [NiFe] hydrogenase from Alteromonas macleodii with unusual stability in the presence of oxygen and high temperature. Appl Environ Microbiol 77(6):1990–1998. doi:10.1128/AEM.01559-10
Vignais PM, Billoud B (2007) Occurrence, classification, and biological function of hydrogenases: an overview. Chem Rev 107:4206–4272. doi:10.1021/cr050196r
Volbeda A, Garcin E, Piras C, de Lacey A, Fernandez VM, Hatchikian EC, Frey M, Fontecilla-Camps JC (1996) Structure of the [NiFe] hydrogenase active site: evidence for biologically uncommon Fe ligands. J Am Chem Soc 118(51):12989–12996. doi:10.1021/ja962270g
Volbeda A, Amara P, Darnault C, Mouesca JM, Parkin A, Roessler MM, Armstrong FA, Fontecilla-Camps JC (2012) X-ray crystallographic and computational studies of the O2-tolerant [NiFe]-hydrogenase 1 from Escherichia coli. Proc Natl Acad Sci USA 109(14):5305–5310. doi:10.1073/pnas.1119806109
Weyman PD, Vargas WA, Chuang RY, Chang Y, Smith HO, Xu Q (2011a) Heterologous expression of Alteromonas macleodii and Thiocapsa roseopersicina [NiFe] hydrogenases in Escherichia coli. Microbiology 157(5):1363–1374. doi:10.1099/mic.0.044834-0
Weyman PD, Vargas WA, Tong Y, Yu J, Maness PC, Smith HO, Xu Q (2011b) Heterologous expression of Alteromonas macleodii and Thiocapsa roseopersicina [NiFe] hydrogenases in Synechococcus elongatus. PLoS One 6(5):e2012. doi:10.1371/journal.pone.0020126
Wu S, Zhang Y (2007) LOMETS: a local meta-threading-server for protein structure prediction. Nucleic Acids Res 35:3375–3382. doi:10.1093/nar/gkm251
Wu S, Zhang Y (2008) MUSTER: improving protein sequence profile-profile alignments by using multiple sources of structure information. Proteins 72:547–556. doi:10.1002/prot.21945
Xu D, Zhang Y (2012) Ab initio protein structure assembly using continuous structure fragments and optimized knowledge-based force field. Proteins 80:1715–1735. doi:10.1002/prot.24065
Yagi T, Kimura K, Daidoji H, Sakai F, Tamura S (1976) Properties of purified hydrogenase from the particulate fraction of Desulfovibrio vulgaris Miyazaki. J Biochem 79(3):661–671
Zhang Y (2008) I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9:40. doi:10.1186/1471-2105-9-40
Zorin NA (1986) Inhibition of Thiocapsa roseopersicina hydrogenase by various compounds. Biokhimiia 51:770–774
Zorin NA, Gogotov IN (1982) Stability of hydrogenase from the purple sulfur bacteria Thiocapsa roseopersicina. Biokhimiia 47(5):827–833
Acknowledgments
We gratefully thank Dr. Artyom Kabanov, Prof. Vladislav Komarov, Dr. Maxim Kondratiev, and Dr. Alexander Samchenko from Institute of Cell Biophysics, Pushchino, for their fruitful advice and help. The work was supported in part by RAS Basic research program “Fundamental principles of nanostructural and nanomaterial technology” and RFBR (Grant Number 14-04-01676).
Conflict of interests
The authors noted in the article title are the only authors of this article. Dr Anatoly Tsygankov and Mr. Azat Abdullatypov report no financial relationships related to any products involved in this study. Dr Anatoly Tsygankov and Mr. Azat Abdullatypov are employees of Institute of Basic Biological Problems of Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdullatypov, A.V., Tsygankov, A.A. Modeling three-dimensional structure of two closely related Ni–Fe hydrogenases. Photosynth Res 125, 341–353 (2015). https://doi.org/10.1007/s11120-014-0071-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-014-0071-z