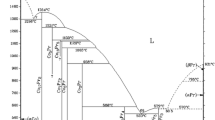
The mixing enthalpies of Al–Pr binary liquid alloys are measured in the ranges 0 < x Pr < 0.15 at 1560 K and 0.46 < x Pr < 1 at 1410–1670 K by isoperibol calorimetry. The Al–Pr binary melts are characterized by significant negative mixing enthalpies: \( \varDelta {H}_{Al- \Pr}^{{}^{min}} \) = –43.1 kJ/mol at x Pr = 0.33 (at 1500 K, extrapolation onto the range of supercooled melts). The activities of components, entropies, Gibbs energies, and liquidus curve of the Al–Pr phase diagram are evaluated using the model of ideal associated solutions.








Similar content being viewed by others
References
L. Jin, Y.-B. Kang, P. Chartrand, and C. D. Fuerst, “Thermodynamic evaluation and optimization of Al–La, Al–Ce, Al–Pr, Al–Nd, and Al–Sm systems using the Modified Quasichemical Model for liquids,” CALPHAD, 35, No. 1, 30–41 (2011).
M. I. Ivanov, V. V. Berezutskii, M. O. Shevchenko, et al., “Thermodynamic properties of Al–Y(La, Eu, Yb) melts,” Dop. Nats. Akad. Nauk Ukrainy, No. 8, 85–90 (2011).
V. G. Kudin, M. A. Shevchenko, I. V. Mateiko, and V. S. Sudavtsova, “Thermodynamic properties of Al–La melts,” Zh. Fiz. Khim., 87, No. 3, 364–370 (2013).
V. I. Kober, I. F. Nichkov, S. P. Raspopin, and A. A. Bogdanov, “Thermodynamic properties of Pr–Al compounds,” Izv. Vuzov. Tsvet. Metall., No. 3, 58–60 (1983).
G. Canneri and A. Rossi, “Alloys of praseodymium and aluminum,” Alluminio, 2, 87–89 (1933).
K. H. J. Buschow and J. H. N. Van Vucht, “Systematic arrangement of the binary rare earth–aluminum systems,” Philips Res. Rep., 22, 233–245 (1967).
K. H. J. Buschow and J. H. N. van Vucht, “The binary systems cerium–aluminum and praseodymium–aluminum,” Z. Metallkd., 57, 162–166 (1966).
A. Saccone, A. M. Cardinale, S. Delfino, and R. Ferro, “Phase equilibria in the rare earth metals (R)-rich regions of the R–Al systems (R = La–Ce–Pr–Nd),” Z. Metallkd., 87, 82–87 (1996).
F. Yin, X. Su, Z. Li, and P. Zhang, “A thermodynamic assessment of the Pr–Al system,” Z. Metallkd., 92, 447–450 (2001).
L. Jin, “Thermodynamic modeling of aluminum–magnesium–rare earth systems,” in: Thesis for the Degree of Philosophy Doctor (Metallurgical Engineering), University of Montreal (2012).
H. Okamoto, “Al–Pr (aluminum–praseodymium),” J. Phase Equilib., 23, No. 4, 381 (2002).
M. E. Drits, E. S. Kadaner, and Nguen Din Shoa, “Solid-state solubility of rare earth metals in aluminum,” Izv. Akad. Nauk SSSR. Metally, No. 1, 219–223 (1969).
L. Rolla, A. Jandelli, G. Canneri, and R. Vogel, “Contribution to the knowledge of the metals and alloys of the rare earths,” Z. Metallkd., 35, 29–42 (1943).
G. N. Zviadadze, L. A. Chkhikvadze, and M. V. Kereselidze, “Thermodynamic properties of binary melts of some rare earth metals with aluminum,” Soobshch. Akad. Nauk. Gruz. SSR, No. 81, 149–152 (1976).
C. Colinet, A. Pasturel, and K. H. J. Buschow, “Molar enthalpies of formation of LnAl2 compounds,” J. Chem. Thermodyn., 17, 1133–1139 (1985).
R. Ferro, G. Borzone, N. Parodi, and G. Cacciamani, “On the thermochemistry of the rare earth compounds with the p-block elements,” J. Phase Equilib., 15, 317–329 (1994).
M. C. Gao, A. D. Rollett, and M. Widom, “Lattice stability of aluminum–rare earth binary systems: A firstprinciples approach,” Phys. Rev. B, 75, 174120/1–174120/16 (2007).
V. V. Efremov, V. I. Kober, V. A. Lebedev, et al., “Thermodynamic properties of aluminum-rich Pr–Al alloys,” Izv. Vuzov. Tsvet. Metall., No. 3, 142–144 (1975).
V. I. Kober, I. F. Nichkov, S. P. Raspopin, and V. M. Kuz’minykh, “Thermodynamic properties of saturated solutions of praseodymium with fusible metals,” in: L. F. Kozin (ed.), Thermodynamics of Metallic Systems [in Russian], Nauka, Alma-Ata (1979), Part. 2, pp. 67–71.
V. I. Kononenko, V. G. Shevchenko, and A. L. Sukhman, “Liquid-phase interaction of praseodymium and aluminum,” Izv. Akad. Nauk SSSR. Metally, No. 6, 85–88 (1979).
G. Borzone, N. Parodi, R. Ferro, et al., “Heat capacity and phase equilibria in rare earth alloy systems. Rrich R–Al alloys (R = La, Pr and Nd),” J. Alloys Compd., 320, No. 2, 242–250 (2001).
C. Deenadas, A. W. Thompson, R. S. Craig, and W. E. Wallace, “Low temperature heat capacities of Laves phase lanthanide–aluminum compounds,” J. Phys. Chem. Solids, 32, No. 8, 1853–1866 (1971).
M. Ivanov, V. Berezutski, and N. Usenko, “Mixing enthalpies in liquid alloys of manganese with the lanthanides,” J. Mater. Res., 102, No. 3, 277–281 (2011).
A. T. Dinsdale, “SGTE data for pure elements,” CALPHAD, 15, No. 4, 319–427 (1991).
M. A. Shevchenko, M. I. Ivanov, V. V. Berezutskii, et al., “Thermodynamic properties of Ni–Sc and Ni–Y alloys,” Zh. Fiz. Khim., 88, No. 6, 909–914 (2014).
M. I. Ivanov, V. V. Berezutskii, M. O. Shevchenko, et al., “Interaction in alloys of europium-containing systems,” Dop. Nats. Akad. Nauk Ukrainy, No. 8, 90–99 (2013).
M. O. Shevchenko, V. G. Kudin, V. V. Berezutskii, et al., “Thermodynamic properties of Al–Sc alloys,” Powder Metall. Met. Ceram., 53, No. 3–4, 243–249 (2014).
V. S. Sudavtsova, M. A. Shevchenko, V. V. Berezutskii, et al., “Thermodynamic properties and phase equilibria in Al (Si)–Ce binary alloys,” Zh. Fiz. Khim., 88, No. 5, 736–746 (2014).
M. O. Shevchenko, V. V. Berezutski, M. I. Ivanov, et al., “Thermodynamic properties of alloys of the binary Al–Sm, Sm–Sn and ternary Al–Sm–Sn systems,” J. Phase Equilib. Diffus., 36, No. 1, 39–52 (2015).
F. R. Boer, R. Boom, W. C. M. Mattens, et al., Cohesion in Metals. Transition Metal Alloys, Elsevier Science Publishers B.V., North-Holland Physics Publishing, Amsterdam (1988), p. 758.
D. S. Kanibolotsky, N. V. Golovataya, O. A. Bieloborodova, and V. V. Lisnyak, “Calorimetric investigation of liquid Al–Ga–Gd alloys,” Thermochim. Acta, 421, 111–115 (2004).
A. Pasturel, C. Chatillon-Colinet, A. Percheron-Guegan, and J. C. Achard, “Thermodynamic study of the valence state of ytterbium in YbAl2 and YbAl3 compounds,” J. Less-Common Met., 90, 21–27 (1983).
Y.-B. Kang, A. D. Pelton, P. Chartrand, and C. D. Fuerst, “Critical evaluation and thermodynamic optimization of the Al–Ce, Al–Y, Al–Sc, and Mg–Sc binary systems,” CALPHAD, 32, No. 2, 413–422 (2008).
G. Cacciamani, S. de Negri, A. Saccone, and R. Ferro, “The Al–R–Mg (R = Gd, Dy, Ho) systems. Part II: Thermodynamic modelling of the binary and ternary systems,” Intermetallics, 11, 1135–1151 (2003).
G. Cacciamani, A. Saccone, S. De Negri, and R. Ferro, “The Al–Er–Mg ternary system. Part II: Thermodynamic modeling,” J. Phase Equilib., 23, No. 1, 38–50 (2002).
L. G. Zhang, H. Q. Dong, G. X. Huang, et al., “Thermodynamic assessment of the Al–Cu–Gd system,” CALPHAD, 33, 664–672 (2009).
H. Bo, L. Liu, X. Xiong, and Z. Jin, “Thermodynamic assessment of the Al–Dy, Dy–Zr, and Al–Dy–Zr systems,” Chin. Sci. Bull., 59, No. 15, 1738–1746 (2014).
T. Tokunaga, H. Kominato, S. Iikubo, and H. Ohtani, “Thermodynamic analysis of phase equilibria in the Mg–Al–Ho ternary system,” Mater. Trans. Jap. Inst. Met., 54, No. 5, 647–655 (2013).
S. Sun, D. Q. Yi, Y. Chen, and C. Wu, “Thermodynamic properties of binary alloys of Al–Er and Si–Er,” Chin. J. Nonferrous Met., 19, No. 9, 1580–1586 (2009).
S. Lin, Z. Nie, H. Huang, et al., “Thermodynamic calculation of Er–X and Al–Er–X compounds existing in Al–Mg–Mn–Zr–Er alloy,” Trans. Nonferrous Met. Soc. China, 20, 682–867 (2010).
V. V. Berezutskii, M. A. Shevchenko, M. I. Ivanov, and V. S. Sudavtsova, “Thermodynamic properties of Ni–Eu and Ni–Yb liquid alloys,” Zh. Fiz. Khim., 88, No. 9, 1297–1306 (2014).
V. S. Sudavtsova, M. I. Ivanov, V. V. Berezutskii, et al., “Thermodynamic properties of Eu–Sn melts,” Zh. Fiz. Khim., 85, No. 12, 2394–2397 (2011).
M. I. Ivanov, N. I. Usenko, V. V. Berezutskii, and N. V. Kotova, Thermodynamics of Binary Melts of Lanthanides with Transition Metals: Monograph [in Ukrainian], Kyiv Derzh. Nats. Univ., Kyiv (2012), p. 87.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkovaya Metallurgiya, Vol. 55, Nos. 1–2 (507), pp. 104–118, 2015.
Rights and permissions
About this article
Cite this article
Shevchenko, M.O., Berezutskii, V.V., Ivanov, M.I. et al. Thermodynamic Properties of Binary Al–Pr Alloys. Powder Metall Met Ceram 55, 78–90 (2016). https://doi.org/10.1007/s11106-016-9783-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-016-9783-2