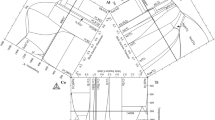
The structure and phase transformations in the Ti–Al–C system were studied by X-ray diffraction, differential thermal analysis, and scanning electron microscopy, including energy-dispersive X-ray spectroscopy and electron backscatter diffraction on samples obtained by arc melting and annealing at high temperatures. The ternary system has a cocrystallization region for the two MAX-phases, N and H. The Ti41.5Al38.5C20 samples contain three phases at all experimental temperatures (from 650 to 1660°C): Ti3AlC2 (N-phase of Ti3SiC2 type), Ti2AlC (H, Cr2AlC type), and binary intermetallic TiAl3 (ε, its own crystal type). The morphology of the as-cast alloy and annealed samples (at temperatures above and below the solidus temperature, 1660 and 1250°C, respectively) shows that invariant solidification at 1405°C (solidus temperature) precedes the univariant simultaneous solidification of N- and H-phases, i.e. both MAX-phases separating from the melt.






Similar content being viewed by others
Notes
* Sc2O3 crucibles, 20 °C/min heating and cooling rate.
References
M. W. Barsoum and T. El-Raghy, “The MAX phases: unique new carbide and nitride materials,” Am. Sci., 89, 334–343 (2001).
P. Eklund, M. Beckers, U. Jansson, et al., “The M n+1AX n phases: materials science and thin-film processing,” Thin Solid Films, 518, No. 8, 1851–1878 (2010).
M. A. Pietzka, Structural Chemistry, Phase Equilibria, and Chemical Analysis in the Systems Ti–Al–C and Ti–Al–N: Thesis [in German], University of Vienna (1992), p. 52.
M. A. Pietzka and J. C. Schuster, “Summary of constitutional data on the aluminum–carbon–titanium system,” J. Phase Equilib., 15, No. 4, 392–400 (1994).
Zh. Ge, K. Chen, J. Guo, et al., “Combustion synthesis of ternary carbide Ti3AlC2 in Ti–Al–C system,” J. Eur. Ceram. Soc., 23, 567–574 (2003).
L. Cornish, G. Cacciamani, D. Cupid, and J. De Keyzer, “Aluminum–carbon–titanium,” in: Landolt-Börnstein, Numerical Data and Functional Relationships in Science and Technology, W. Martinsen (ed.), New Series. Group IV: Physical Chemistry, G. Effenberg and S. Ilyenko (eds.), Ternary Alloy Systems, Phase Diagrams, Crystallographic and Thermodynamic Data Critically Evaluated by MSIT, Vol. 11E1, Springer-Verlag, Berlin, Heidelberg (2009), pp. 41–71.
P. Villars and L. D. Calvert, Pearson’s Handbook of Crystallographic Data for Intermetallic Phases, 2nd ed., Handbook in 4 Vols., ASM Int. Mater. Park, Ohio (1991).
V. T. Witusiewicz, A. A. Bondar, U. Hecht, et al., “The Al–B–Nb–Ti system. III. Thermodynamic reevaluation of the constituent binary system Al–Ti,” J. Alloys Compd., 465, No. 1–2, 64–77 (2008).
J. Braun and M. Ellner, “Phase equilibria investigations on the aluminum-rich part of the binary system Ti–Al,” Met. Mater. Trans. A, 32A, 1037–1048 (2001).
V. T. Witusiewicz, A. A. Bondar, U. Hecht, et al., “Experimental study and thermodynamic remodeling of the ternary Ti–Al–C system,” in: Proc. Discussion Meeting on Thermodynamics of Alloys (TOFA), Pula, Croatia (2012), p. 2.
M. Pirani and H. Alterthum, “On the method for determining the melting point of refractory metals,” Z. Elektrochem., 29, No. 1–2, 5–8 (1923).
R. A. Young, A. Sakthivel, T. S. Moss, and C. O. Paiva-Santos, “DBWS-9411—an upgrade of the DBWS programs for Rietveld refinement with PC and mainframe computers,” J. Appl. Crystallogr., 28, 366–367 (1995).
Ju. A. Kocherzhinsky, “Differential thermocouple up to 2450°C and thermographic investigations of refractory silicides,” in: Proc. Third ICTA (Davos) Therm. Analysis, Birkhäuser Verlag, Basel (1971), Vol. 1, pp. 549–559.
Yu. A. Kocherzhinsky, E. A. Shishkin, and V. I. Vasilenko, “DTA apparatus with a thermocouple sensor to 2200°C,” in: Phase Diagrams of Metallic Systems [in Russian], Nauka, Moscow (1971), pp. 245–249.
W. J. Boettinger, U. R. Kattner, K.-W. Moon, and J. H. Perepezko, DTA and Heat-flux DSC Measurements of Alloy Melting and Freezing: NIST Recommended Practice Guide, Special Publication 960-15, National Institute of Standards and Technology, Washington, USA (2006), p. 90.
J. Grobner, H. L. Lukas, and F. Aldinger, “Thermodynamic calculations in the Y–Al–C system,” J. Alloys Compd., 220, 8–14 (1995).
L. F. S. Dumitrescu, M. Hillert, and B. Sundman, “A reassessment of Ti–C–N based on available assessments of Ti–N and Ti–C,” Z. Metallkd., 90, No. 7, 534–541 (1999).
V. T. Witusiewicz, B. Hallstedt, A. A. Bondar, et al., “Thermodynamic description of the Al–C–Ti system,” J. Alloys Compd., 623, 480–496 (2015).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkovaya Metallurgiya, Vol. 54, Nos. 7–8 (504), pp. 111–124, 2015.
Rights and permissions
About this article
Cite this article
Sleptsov, S.V., Bondar, A.A., Witusiewicz, V.T. et al. Cocrystallization of Max-Phases in the Ti–Al–C System. Powder Metall Met Ceram 54, 471–481 (2015). https://doi.org/10.1007/s11106-015-9738-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-015-9738-z