Abstract
The CRISPR/Cas9 system is a site-specific genome editing tool that has been widely used in various plant species. The plant virus-based gRNA (guide RNA) delivery system, which differs from the typical Agrobacterium-mediated transformation, is an attractive method to facilitate the application of CRISPR/Cas9. The virally delivered gRNA is usually driven by heterologous plant U6 or viral promoters (e.g., pea early-browning virus, PEBV; barley stripe mosaic virus, BSMV). However, heterologous promoters may have poor performance in some cases. In this paper, a feasible option to detach gRNA(s) from the virus genome is employed. Specifically, the Csy4-RNA processing system is used to release gRNA(s) from the tobacco rattle virus (TRV). The coding sequences of Cas9 and Csy4 nucleases are cloned into a single polycistronic expression cassette under an estrogen-inducible promoter in a binary vector, and the gRNA is cloned into the TRV genome flanked by two 20 bp Csy4 recognition sites. The results show that the Csy4-processing TRV-based delivery system works effectively in targeting single and multiple sites, nucleotide replacement, and large fragment deletion in Cas9-mediated genome editing via transient expression in Nicotiana benthamiana. The Csy4-TRV is a promising gRNA(s) processing and delivery system for CRISPR/Cas9 genome editing. This method can be easily adapted to other plant RNA viruses, facilitating the application of the CRISPR/Cas9 system in plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The CRISPR/Cas9 system is a site-specific gene editing tool that has been widely used in various eukaryotes, including plants (Wang and Doudna 2023). The system typically consists of two components: Cas9 and guide RNA (gRNA). The Cas9 component is usually expressed under the control of different polymerase II (Pol II) promoters, such as the constitutive cauliflower mosaic virus (CaMV) 35S promoter, the maize ubiquitin promoter (Karmacharya et al. 2023), the estrogen-inducible promoter (Tang et al. 2016), the light-inducible promoter (Polstein and Gersbach 2015), or tissue-specific promoters (e.g., pYAO) (Ai et al. 2023). The mRNAs produced by these promoters undergo extensive processing, modification, and nuclear-cytoplasmic transport. Therefore, Pol II promoters are not suitable for directly driving gRNA expression in the CRISPR/Cas9 system. The gRNA component is commonly expressed under the control of a U6 or U3 polymerase III (Pol III) promoter (Massel et al. 2022). However, many plant species lack well-characterized Pol III promoters, and the heterologous expression of these promoters often has uncertain effects. This limits the application of the CRISPR/Cas9 system in many species.
To overcome the limitation of the Pol III promoter, a gRNA expression cassette with an RNA-processing system under the Pol II promoter has been developed. The RNA-processing system consists of ribozymes (Oh et al. 2021), tRNA-processing (Xie and Yang 2019), or Csy4-RNA processing system (Nissim et al. 2014). In these systems, the gRNA driven by the Pol II promoter is fused to a ribozyme sequence, a tRNA sequence, or a Csy4 recognition sequence (C4 site), respectively. The mature gRNA is then released from the primary mRNA transcripts by self-cleavage ribozymes, endogenous tRNAs (RNase P or Z), or Csy4 nuclease. This method removes the additional bases at the 5′ and 3′ ends of gRNA, which can adversely affect Cas9-mediated mutagenesis (Haurwitz et al. 2010). Moreover, this approach eliminates the further modification of gRNA after expression via the Pol II promoter. Another benefit of these strategies is that they facilitate the assembly of multiple gRNAs in a single transcript-expressing cassette to enhance the multiplex targeting capability.
The CRISPR/Cas9 system consists of components that need to be delivered efficiently into cells for genome editing. In plants, a common method of delivery is Agrobacterium-mediated T-DNA transformation, which involves cloning Cas9 and gRNA into a binary vector (Cody et al. 2017). Another possible strategy is virus-based delivery, which integrates gRNA into the virus genome and delivers it into newly developed plant cells through systemic viral infection (Ali et al. 2015; Liu and Zhang 2020; Yin et al. 2015). To express and release the mature gRNA from the virus genome, different promoters have been used to drive gRNA expression in the geminivirus or RNA virus-based vector, such as the heterologous plant U6 promoter (Yin et al. 2015) or the pea-early browning virus (PEBV) promoter (Ali et al. 2015).
The ability of a virus to infect a potential plant is essential for the function of a virus-based delivery system. To accommodate different host plants, various viral constructs need to be developed. This study aimed to use the Csy4-RNA processing system to release gRNA from the genome of tobacco rattle virus (TRV), a well-studied plant RNA virus, and to carry multiple gRNAs with this system. The TRV infectious clone with the CaMV 35S promoter was modified to integrate one or more gRNAs, and the Csy4 recognition sites were inserted around the gRNA region. These TRV-based vectors with gRNA were transferred into Nicotiana benthamiana (N. benthamiana) by agroinfiltration. A Cas9 expression construct containing both Cas9 and Csy4 nucleases was also expressed in the N. benthamiana leaves that were systemically infected by the TRV-gRNA system through agroinfiltration. The results showed that the Csy4-processing TRV-based CRISPR/Cas9 genome editing system could successfully edit the host genome and perform nucleotide substitution and large fragment deletion.
Experimental Procedures
Vector Construction
Figure S1 shows all the primers used in this study. A PCR was performed with primers NbPDSVIGS-BamH1-F and NbPDSVIGS-Xho1-R using N. benthamiana cDNA as a template to obtain the VIGS (virus-induced gene silencing) plasmid. The PCR products were digested by BamH1 and Xho1 and inserted into the same digested pTRV2 (Liu et al. 2002), resulting in pTRV2-VIGS (Fig. S3). To develop the C4 sites flanking gRNA for targeting the first locus of the PDS gene with pTRV2, a PCR was performed using primers 20gKam-EcoR1-F and 20gRNA-Xba1-R with pHSN6A01 (Xing et al. 2014) as a template. The PCR products were digested by EcoR1 and Xba1 and inserted into the same digested pTRV2-VIGS, generating pTRV2-VIGS-g1PDS (Fig. 1B). To develop the C4 sites flanking gRNAs for targeting the first and second place of the PDS gene with pTRV2, a PCR was performed using primers 20g2Ka-Xho1-F and 20gRNA-Xma1-R with pHSN6A01 as a template. The PCR products were digested by Xho1 and Xma1 and inserted into the same digested pTRV2-VIGS-g1PDS, producing pTRV2-VIGS-g1g2PDS (Fig. 1B). Furthermore, the same method can be applied to develop the C4 sites flanking other interested gRNAs for targeting interested genes with pTRV2. That is, a PCR can be performed using primers that integrate the 20 bp C4 recognition sequence and the restriction enzyme sequence in the MCS of pTRV2 with pHSN6A01 or other plasmids containing the guide RNA sequence as a template. The PCR products can be digested by the corresponding restriction enzymes and inserted into the same digested pTRV2 (Fig. 1B). To develop the ribosomes flanking gRNA with pTRV2, a PCR was performed using primers gKam-HH (EcoR)-F0 and gRNA-HDR (Xba)-R with pHSN6A01 as a template. The PCR products were digested by EcoR1 and Xba1 and inserted into the same digested pTRV2-VIGS, resulting in pTRV2-VIGS-RzgPDS (Fig. 1B). To develop the C4 sites flanking gRNAs for targeting EPSPS sequence with pTRV2, a PCR was performed using primers 20gPHTIP1-EcoR1-F and 20gRNA-Xba1-R with pHSN6A01. The resulting PCR products were digested by EcoR1 and Xba1 and inserted into pTRV2-VIGS that had been digested by the same enzymes, generating pTRV2-VIGS-g1EPS. Next, PCR was performed with pHSN6A01 as a template and primers 20gPHTIP2-Xho1-F and 20gRNA-Xma1-R. The resulting PCR products were digested by Xho1 and Xma1 and inserted into pTRV2-VIGS-g1EPS that had been digested by the same enzymes, generating pTRV2-VIGS-g1g2EPSPS.
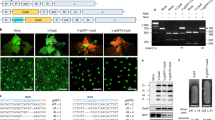
gRNA(s) carried by the TRV vector. A Schematic representation of pTRV1 and pTRV2. Rz denotes a ribozyme. Red arrows indicate the positions of primers used for detecting virus infection by RT-PCR analysis. B Schematic representation of pTRV2 derivatives. DNA fragments were inserted into pTRV2. C4 refers to the Csy4 recognition site. Red arrows indicate the positions of primer “gR” used for detecting gRNA integration into the TRV2 genome by RT-PCR analysis. C The photobleaching phenotype induced by TRV derivatives at 12 days after inoculation. Experiments were repeated at least three times. D RT-PCR analysis of mRNAs in virus-infected plants. EF1a was used as a control. The primers 1F and 1R were used for detecting TRV1; 2F and 2R for TRV2; 3F and gR for testing gRNA integration into the TRV2 genome. The band of 1F1R in lane 1 is smaller than the expected band and corresponds to primer dimers. Lane M is the DNA ladder. Lanes 1 to 4 are the RT-PCR bands of which the PCR template cDNA was transcribed from “Mock,” “TRV:VIGS,” “TRV:VIGS-g1PDS,” and “TRV: VIGS-Rzg1PDS”
A plasmid pMDC7:Cas9-p2a-Csy4 that expresses Cas9 and Csy4 inducibly was constructed by Golden Gate Assembly and Gateway transfer. Six plasmids were prepared as modules for Golden Gate Assembly. The first module plasmid was obtained by inserting the PCR amplicons from pEarleyGate101 into the pGEM-T easy vector, using the primers A-dummy-YFP-F and nYFP-R. The second module plasmid was pGGB003 (Lampropoulos et al. 2013). The third module plasmid was obtained by inserting the PCR amplicons from a variant of plasmid pHSN6A01 containing mutations A10D and A841H into the pGEM-T easy vector, using the primers Cas9-(C)-F and Cas9-(D)-R. The fourth module plasmid was obtained by inserting the PCR amplicons from no template into the pGEM-T easy vector, using the overlapping primers P2A-(D)-F0 and P2A-(E)-R0. The fifth module plasmid was obtained by inserting the PCR amplicons from plasmid PGK1p-Csy4-Pa (Nissim et al. 2014) into the pGEM-T easy vector, using the primers Csy4-(E)-F01 and Csy4-(F)-R. The sixth module plasmid was obtained by inserting the PCR amplicons from pMDC32 (Curtis and Grossniklaus 2003) into the pGEM-T easy vector, using the primers NOS-(F)-F and NOS-(G)-R. All six-module plasmids were assembled into pGGZ001 (Lampropoulos et al. 2013) by Golden Gate Assembly (Fig. S4). The assembly reaction consisted of mixing 0.5 µl of each module plasmid (150 ng/µl each) with 0.5 µl of pGGZ001 (150 ng/µl), 0.5 µl of BSA, 0.3 µl of Bsa1-HF (NEB, R3535L), 0.3 µl of T4 ligase (NEB, M0202M), and 0.5 µl of T4 ligase buffer in a total volume of 5 µl. The mixture was subjected to 50 cycles of PCR at 37 °C for 5 min and 16 °C for 5 min, followed by 50 °C for 5 min and 80 °C for 10 min. The reaction products were transformed into DH5α competent cells for further clone screening. The six-module sequences (Fig. S4) were amplified with primers Z001-attB1-F0 and Z001-attB2-R0. The PCR products were inserted into pDONR207 by BP reaction with the BP Clonase II Enzyme Mix (Thermo Fisher, 11,789,020) and then transferred to pMDC7 (Curtis and Grossniklaus 2003) by LR reaction with the LR Clonase II Enzyme Mix (Thermo Fisher, 11,791,020) to produce the estrogen inducible vector pMDC7: Cas9-p2a-Csy4.
The EPSPS-targeting plasmid was constructed by amplifying a portion of the EPSPS fragment with primers EPSPS-F and EPSPS-R, using common bean (Phaseolus vulgaris) cDNA as a template. The PCR amplicons were cloned into pDONR207 by BP reaction and sequenced. The EPSPS fragment was then shuttled into pEarleyGate301 by LR reaction. The EPSPS repair template plasmid (Fig. S5) was constructed by an overlapping PCR technique. In the first round of PCR, fragment 1 and fragment 2 were amplified with primers PhTIPS-ov-F1/PhTIPS-ov-R1 and PhTIPS-ov-F2/PhTIPS-ov-R2, respectively, using the EPSPS-targeting plasmid as template. In the second round of PCR, fragments 1 and 2 were overlapped with primers PhTIPStemp-(A)-F and PhTIPStemp-(G)-R. The overlapping PCR products were inserted into pGZZ001 by Golden Gate Assembly, generating pGZZ001-template. PCR was performed with primers Z001-attB1-TIPF0 and Z001-attB2-TIPR0, using pGZZ001-template as a template. The PCR products were cloned into pDONR207 by BP reaction and transferred into pMDC99 by LR reaction, producing the EPSPS repair template plasmid. All the constructs were verified by Sanger sequencing.
Virus Infection and Leaf Infiltration
N. benthamiana were infected with TRV-based vectors via agroinfiltration. The plasmids pTRV1, pTRV2, and their derivatives were electroporated into Agrobacterium tumefaciens EHA105 strain. The selected single clone was cultured in an LB liquid medium with shaking (220 rpm) at 28 °C overnight. The culture was harvested, centrifuged, and washed twice with the infiltration buffer (10 mM MgCl2, 100 µM acetosyringone). The cells were resuspended in the same buffer to a final OD600 = 0.5. The Agrobacterium cells carrying pTRV1 and pTRV2 or their derivatives were mixed at a 1:1 ratio, incubated for 2 h at room temperature, and then co-infiltrated into the leaf surface of N. benthamiana with a 1-ml syringe. Agrobacterium cells without any plasmid were used as the control. Cas9 and Csy4 plasmid were transiently expressed in N. benthamiana that were systemically infected with the virus. The agroinfiltration procedure was identical to the one used for virus infection. To induce Cas9 and Csy4 expression, 5 µm of 17-β-estradiol (Sigma, E8875) was added to the infiltration buffer. Each leaf infiltration was repeated at least three times, using a different plant for each replicate.
RNA Extraction and RT-PCR Analysis
Total RNA from plants was extracted using the Plant/Fungi Total RNA Purification Kit (Norgen, 31,350) following the manufacturer’s protocol for RNA analysis. The 5 × iScript cDNA kits (Bio-rad, 1,708,890) were used to synthesize cDNA. The 2X PCR TaqMasterMix with dye (abm, G013-dye) was used according to the manufacturer’s instruction for RT-PCR analysis. The housekeeping gene EF1a served as a control. TRV1 was detected by primer pair 1F and 1R. TRV RNA2 was detected by primer pair 2F and 2R. The gRNA-integrated TRV RNA2 was detected by primer pair 3F and gR. The primer sequences are shown in Fig. S1.
Genome Editing Analysis
Genome editing analysis was conducted according to the method described by Nekrasov et al. (2013), with some modifications. Genomic DNA was extracted from plants using the DNeasy Plant Mini Kit (QIAGEN, 69,106). High-fidelity PCR was performed with Phusion High-Fidelity DNA Polymerase (NEB, M0530S), using genomic DNA with or without restriction enzyme digestion as a template. The PCR primers PDS-(A)-F and PDS-(G)-R, which flank the PDS-targeting sites, were used to detect PDS mutagenesis. The primers EPS-(A)-F and pEarly-(G)-R, which flank the EPSPS-targeting sites in the targeting plasmid, were used to detect EPSPS mutagenesis. The PCR products were digested by corresponding restriction enzymes and analyzed by gel agarose electrophoresis for small indels (insertion and deletion) and substitution mutation test. For large deletion mutation tests, the PCR products were directly analyzed by gel electrophoresis. The expected bands were purified by QIAquick Gel Extraction Kit (QIAGEN, 28,706) and inserted into pGGZ001 by Golden gate cloning. Sanger sequencing was performed using the primers Z001-F and Z001-R to verify mutagenesis in DNA fragments. All mutagenesis assays were independently repeated at least three times. ImageJ (version 1.8.0) was used to measure the mutation rate. Off-target loci were selected and tested based on a previous report (Nekrasov et al. 2013). Genomic DNA was extracted from N. benthamiana leaves that were infected with TRV:VIGS-g1PDS and expressed pMDC7:Cas9-p2a-Csy4. MlyI digested the genomic DNA. PCR bands of the expected size were obtained by amplifying nine potential off-target loci from the MlyI-digested genomic DNA. Agarose gel electrophoresis analyzed the PCR amplicons of these nine loci after further digestion by MlyI. Figure S1 shows the locus number and PCR primer pair of each locus.
Results
Infectivity of gRNA-Integrated TRV
Previous studies have reported plant virus-based delivery systems that express and release gRNA from the virus genome by a heterologous promoter during Cas9-mediated genome editing (Ali et al. 2015; Yin et al. 2015). This study employed a Csy4-RNA processing system (Nissim et al. 2014) consisting of two RNA genomes (TRV1 and TRV2) to release the gRNA from TRV. A gRNA targeting the phytoene desaturase (PDS) gene, flanked by two 20 base pair (bp) C4 sites, was cloned into the multiple cloning site (MCS) of pTRV2 (Liu et al. 2002), a vector derived from TRV RNA2 (Fig. 1A), to evaluate the delivery capacity of gRNA-carrying-TRV. A 102 bp PDS fragment was also inserted into the construct to induce a photobleaching phenotype (Velásquez et al. 2009) through virus-induced gene silencing (VIGS) of PDS, serving as a visible infection indicator. This construct and pTRV1 (Liu et al. 2002), a vector derived from TRV1 (Fig. 1A), were agroinfiltrated into N. benthamiana (marked as TRV:VIGS-g1PDS, Fig. 1B). The photobleaching phenotype (induced by VIGS of PDS) was observed 2 weeks after infection. Two more weeks later, the TRV genomes TRV1 and TRV2 were detected by RT-PCR in newly developed leaves. The timing and appearance of the photobleaching phenotype, as well as the virus RNA abundance in TRV:VIGS-g1PDS-infected plants, were similar to those in TRV:VIGS infected plants (TRV vector only carrying the 102 bp PDS fragment for VIGS silencing, Fig. 1B, C, and D). These results indicate that the C4 sites flanking the gRNA did not interfere with the movement and infection of TRV in N. benthamiana.
To release the gRNA from TRV, ribozymes were also employed (Fang et al. 2017). The gRNA was flanked by two ribozymes, a Hammerhead (HH) ribozyme at the 5′ end and a hepatitis delta virus (HDV) ribozyme at the 3′ end, and cloned into the 102 bp PDS fragment containing pTRV2 (Fig. 1B). The construct, along with pTRV1, was delivered into N. benthamiana (marked as TRV:VIGS-Rzg1PDS). The infected plants showed a photobleaching phenotype on their leaves (Fig. 1C). However, unlike the plants infected by TRV:VIGS or TRV:VIGS-g1PDS, which exhibited highly uniform white leaves, the leaves infected by TRV:VIGS-Rzg1PDS had dispersed white spots, and this phenotype appeared 6 to 8 days later than the uniform white leaves. A weaker band was detected by RT-PCR for the gRNA-integrated TRV RNA2 in TRV:VIGS-Rzg1PDS-infected leaves compared to TRV:VIGS-g1PDS (Fig. 1D). This reduced gRNA integration suggested that the ribozyme sequences (Fig. 1A) affected the TRV stability or movement. Based on these results, the Csy4 processing system was chosen for further experiments instead of the ribozyme system.
Genome Editing with Csy4-Released gRNA from TRV
To assess the genome editing effect of gRNA released by Csy4 from TRV, a Cas9 and Csy4 nuclease-expressing construct (pMDC7:Cas9-p2a-Csy4) was developed. Cas9 and Csy4 were cloned into a single-polycistronic binary vector (Fig. 2A) to coordinate the expression of these two nucleases. A 2A self-cleaving peptide (P2A) sequence, which was originally identified and characterized in the foot-and-mouth disease virus (FMDV) (Zetsche et al. 2015), separated the coding sequence of Cas9 and Csy4. Moreover, an estradiol inducible promoter was employed to drive the expression of Cas9 and Csy4 for precise control of genome editing. The construct was agroinfiltrated into TRV:VIGS-g1PDS-infected N. benthamiana leaves. Genomic DNA was extracted 2dpi from leaves for Cas9-induced mutation detection. PCR amplicons from the region flanking the PDS target locus were digested by the MlyI restriction enzyme and analyzed by agarose gel electrophoresis. A MlyI-resistant band appeared in the samples whose expression of Cas9 and Csy4 was induced by estradiol (Fig. 2B), indicating that mutations were induced by Cas9 and Csy4 expression. The mutations were resistant to MlyI digestion due to the loss of the restriction site, which resulted from Cas9-mediated non-homologous end joining (NHEJ) (Shan et al. 2014). The mutagenesis frequency was 3.9% (Fig. 2B). The band resistant to MlyI was cloned and sequenced. The sequencing of nine individual clones showed the existence of five kinds of deletions and one kind of insertion (Fig. 2C, F). These findings demonstrate that the Csy4-processing TRV-based CRISPR/Cas9 genome editing system is functional in N. benthamiana cells. A previous method (Nekrasov et al. 2013) was used to test the off-targeting and no off-target mutations were detected (Fig. S2). The Csy4-processing TRV-gRNA delivery system is likely to have the same off-target activity as the gRNA expressing system controlled by Pol III promoter (such as U6) since there are no changes in gRNA scaffold sequences.
Csy4-processing TRV-based genome editing. A Diagram of genome editing. Cas9 and Csy4 expression is induced by estradiol. Csy4 cleaves C4 sites in TRVRNA2 and releases gRNA, which guides Cas9 for genome editing. B Genome editing on the PDS locus. Plants with “TRV:VIGS-g1PDS” were agroinfiltrated with pMDC7:Cas9-p2a-Csy4. Lane M is the DNA ladder. Lanes 1 (+ estradiol) and 3 (-estradiol) show the MlyI-digested PCR products. Lane 2 shows the PCR product without MlyI digestion. The mutation rate was calculated by dividing the intensity of the uncut band by the total intensity of all bands in Lane 1. The MlyI-resistant band is indicated by the red arrow. The mutagenesis assays were repeated independently at least three times. C Sequencing results of indels in PDS. The band indicated by the red arrow in (B) was cloned and sequenced. The wild-type sequence is shown at the top. The target site (yellow), PAM (red), and the mutations (red letters) are indicated. The length of insertions ( +) and deletions ( −), and the frequency with which each DNA sequence occurred are presented at right (in parentheses). D A large deletion in the PDS locus was confirmed by Sanger sequencing. The band indicated by the red arrow in (E) was cloned and sequenced. Two PAM sequences are shown in red and green. The deletion is underlined in red. The size of the deletion is shown on the right of the sequences. The frequency of each DNA sequence is presented in parentheses. E A large deletion in the PDS locus was induced by agroinfiltration of the “TRV:VIGS-g1g2PDS” plants with pMDC7:Cas9-p2a-Csy4. PCR products using MlyI-digested genomic DNA as a template are shown in lanes 1(− estradiol) and 2 (+ estradiol). The PCR amplicons with a large deletion are marked by the red arrow. F Chromatograms of WT and edited sequences in (C) are shown. Two types of mutations are observed. The locations of gRNA and MlyI sites are indicated. The mutated nucleotide bases are marked by red arrows
Targeting Multiple Sites Through Csy4-Released gRNAs from TRV
To evaluate the capacity of the Csy4-processing TRV-based CRISPR/Cas9 genome editing system to deliver multiple gRNAs, two gRNAs targeting different loci of the PDS sequence were inserted into the MCS site of pTRV2 (Fig. 1B). The gRNA only needs to be flanked by two 20 bp C4 sites at its 5′- and 3′-ends, which facilitates manipulation. The construct along with pTRV1 was agroinfiltrated into N. benthamiana (marked as TRV:VIGS-g1g2PDS). The systemically infected leaves were then agroinfiltrated with pMDC7: Cas9-p2a-Csy4. To enrich for Cas9-induced mutations, the genomic DNA was digested by MlyI, which recognizes one target of gRNAs in wild-type PDS (Fig. 2D). The MlyI-digested genomic DNA was used as a template to amplify the selected targets in PDS fragments. A small band was amplified from the samples expressing Cas9 and Csy4 (Fig. 2E). It was then gel purified and cloned for Sanger sequencing verification. The results showed PDS amplicons had a large deletion between the two gRNA targets (Fig. 2D). These results indicate that the Csy4-processing TRV-based system can efficiently deliver multiple gRNAs for Cas9-mediated genome editing.
Gene Replacement Through Csy4-Released gRNAs from TRV
The Csy4-processing TRV-based CRISPR/Cas9 genome editing system was also used to perform gene replacement, and the 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) gene was selected as the test target. It has been reported that a double amino acid substitution (T102I + P106S, named as TIPS mutation) in the conserved motif in the second exon of EPSPS confers glyphosate herbicide resistance (Zhang et al. 2016). A fragment of EPSPS was cloned into a binary vector as the target plasmid in this study. Two EPSPS-targeting gRNAs flanked by C4 sites were inserted into pTRV2 (Fig. S3). In addition, a donor plasmid was constructed, containing the TIPS-mutated second exon sequence, the mutated first and second intron sequence, and target 1 and target 2 sequence flanked at 5′ and 3′-ends, respectively (Figs. 3A and S5). The donor template (the sequence between targets 1 and 2 in the donor plasmid, Fig. 3A) could be released from the donor plasmid during genome editing. The TRV vectors carrying EPSPS targeting gRNAs were agroinfiltrated into N. benthamiana leaves (TRV:VIGS-g1g2EPSPS). The pMDC7:Cas9-p2a-Csy4 construct, along with target and donor plasmids were then agroinfiltrated into the systemically infected leaves. Two days after infection, the leaf samples were collected and the genomic DNA was extracted. The enrichment of Cas9-induced mutations was assessed by genomic DNA digestion with BsrDI, which recognizes a site between two targets in wild-type EPSPS (Fig. 3B, C). Primers flanking the target sites were used to amplify the EPSPS fragment from the BsrDI-digested genomic DNA. A small PCR band was observed in the samples expressing Cas9 and Csy4 upon estradiol induction, but not in the negative control or without estradiol treatment (Fig. 3D). This band was purified from the gel and cloned for sequencing verification. The results revealed a large deletion in the EPSPS fragments (Fig. 3B). The PCR products were further subjected to BsrDI digestion and agarose gel electrophoresis, which showed that some of the PCR products were resistant to BsrDI (Fig. 3E). This BsrDI-resistant band was cloned and verified by Sanger sequencing. The results indicated that gene substitutions, including TIPS mutation, had occurred (Fig. 3CF). The large deletion was also detected in some samples (Fig. 3E). The ratio of gene substitution to large deletion was 1:2.1. These results demonstrated that the Csy4-processing TRV-based CRISPR/Cas9 genome editing system could be applied for nucleotide replacement as well as large fragment deletion.
Gene replacement by HDR using the Csy4-processing TRV system. A Schematic representation of gene replacement in EPSPS by HDR. Targets 1 and 2 and their corresponding mutated versions are indicated. Four HDR arms are shown. The TIPS mutation is in the second exon and eliminates the BsrDI site in the target. B Sequencing results of a large deletion in EPSPS. The red arrow points to the band in (B) that was confirmed by sequencing. The target sites are highlighted in yellow, two PAMs are highlighted in red and green, and the position of the BsrDI site is marked. The deletion is shown as a red dashed line. The length of deletions (bp) and the frequency of each DNA sequence are presented on the right (in parenthesis). C The sequences of different HDR types. The green arrow points to the band in (C) that was confirmed by sequencing. The TIPS mutation is highlighted in purple. The mutated targets 1 and 2 in the donor template are shown in the red box and green box, respectively. The mutation is in red text. D A large deletion in EPSPS. PCR products were obtained from MlyI-digested genomic DNA of “TRV:VIGS-g1g2EPSPS” plants agroinfiltrated with pMDC7: Cas9-p2a-Csy4. The red arrow indicates PCR amplicons with large deletion, as shown in lanes 1 (− estradiol) and 2 (+ estradiol). E Gene replacement in EPSPS. BsrDI-digested PCR products of lanes 1 and 2 in (B) are shown in lanes 1 and 2, respectively. The red arrow indicates DNA fragments with a large deletion. The green arrow indicates BsrDI-resistant DNA fragments containing the TIPS mutation. The relative efficiency of gene replacement compared to large deletion (1:2.1) was calculated by the ratio of the intensity of the green arrow band to the red band in lane 2. F Chromatogram of WT and edited sequences in (C). Two types of mutations (Mut) are shown. The location of BsrDI sites is indicated. Red arrows indicate the mutated nucleotide bases
Discussion
The CRISPR/Cas9 system is a powerful tool for gene function analysis and trait improvement in many plants. To deliver the CRISPR/Cas9 components into the host plant, a plant virus-based gRNA delivery system can be used (Yin et al. 2015). This strategy requires the release of the gRNA(s) from the virus. This study presents a novel approach to process gRNA-integrated TRV using Csy4 nuclease. The expression of Cas9 and Csy4 is regulated by an estrogen-inducible promoter (Curtis and Grossniklaus 2003), allowing precise control of genome editing. Furthermore, Cas9 and Csy4 are co-expressed in a single polycistronic system, ensuring their simultaneous and coincident induction by estrogen (Zetsche et al. 2015). Cas9 and Csy4 are nucleases that originate from the bacterial defense system, where there is no distinction between the nucleus and the cytoplasm. To enable their function in plant cells with a nuclear envelope, Cas9 was fused with a nuclear localization signal (NLS) sequence in the construct, ensuring its delivery into the plant cell nucleus. Csy4, on the other hand, has a low molecular weight (around 25 kDa) and does not require NLS fusion (Nissim et al. 2014). It can shuttle through nuclear pore complexes (NPCs) by free diffusion (Terry et al. 2007), allowing it to access both the cytoplasm and the nucleus. Thus, the construct could process not only cytoplasmic RNA viruses, such as TRV (Yin et al. 2015), but also nuclear RNA transcripts derived from DNA virus genomes. In this study, the Csy4-TRV were applied in N. benthamiana. However, given the broad host range of TRV, we recognize that this technique has potential applications in other TRV host plants, including some important crops, such as tomato and cucumber. TRV is a plant viral vector widely used for VIGS experiments, and this study used it to conduct genome editing. Compared with the transient VIGS technique, the biggest advantage of the genome editing technique is that it can alter the DNA sequence itself rather than affecting the gene expression level by changing the RNA abundance. Therefore, even if the viral vector disappears, the altered DNA sequence will not revert. Furthermore, the genome editing technique can modify the target site more accurately than VIGS.
C4 sites were added to both sides of the gRNA sequence in TRV-based constructs. The Csy4 processing system has several advantages. First, the small size of the C4 site (20 bp) simplifies the cloning manipulation, as primers can be easily designed with the C4 site during construction without modifying the viral vector, which is usually restricted by the size of the delivered cargo (Cheuk and Houde 2018). Second, the gRNA release by Csy4 can be controlled by an inducible or constitutive promoter (such as XVE or 35S promoter), avoiding the need for organism-specific heterologous promoters to release gRNA. This strategy can be applied to other RNA viruses, in case the heterologous promoters may not work effectively in some cases or plant species. Third, a previous study reported that the gRNA processing by Csy4 has a much higher Cas9-induced mutation efficiency compared with the gRNA expressed from Pol III promoters (Čermák et al. 2017). In this study, ribozymes were also used to release gRNA from TRV. However, the results showed that the infection efficiency of ribozyme-integrated TRV was impeded (Fig. 1C, D). The result is consistent with the report (Cody et al. 2017) showing that the ribozyme negatively affects the replication of another plant virus, tobacco mosaic virus (TMV).
Several plant geminiviruses have been engineered as replicon-based systems to express the components of the CRISPR/Cas9 system, such as bean yellow dwarf virus (BeYDV) in Nicotiana tobacum cells (Baltes et al. 2014), wheat dwarf virus (WDV) in wheat cells (Gil‐Humanes et al. 2017), and rice (Wang et al. 2017). These DNA replicons, which contained the CRISPR/Cas9 components, were transiently amplified to high copy numbers in plant cells, resulting in the enrichment of CRISPR/Cas9 components and the enhancement of gene targeting frequencies. However, these replicons lost the ability to infect cells since the genes essential for cell-to-cell movement were replaced by heterologous sequences (Gil‐Humanes et al. 2017). In contrast to the replicon-based system, this study used a gRNA that was carried by the TRV virus and had the ability to infect and deliver the gRNA to newly developed cells. The viral movement in the plant was beneficial for gRNA delivery in CRISPR/Cas9 editing, especially for plant species that were resistant to transformation by traditional Agrobacterium-mediated T-DNA methods (Yin et al. 2015). Cas9 was transiently expressed here, which likely led to low editing efficiency (3.9% for single gRNA). The editing efficiency ranged from 0.5 to 5%, depending on the target and the experiment. To perform PCR, the genome DNA template was enriched, since the efficiency of multiplexing gRNAs and gene replacement was even lower than that of the single gRNA. This low efficiency in the transient system agreed with a previous report of 1.9% (Nekrasov et al. 2013). The editing frequency might be increased in stably transformed plants, in which Cas9 could be overexpressed as indicated in other studies (~ 50%) (Ali et al. 2015; Yin et al. 2015). In this study, gene editing was not detected in the offspring. It was hypothesized that to stably inherit gene editing to the next generation using the method of virus delivery of gRNA, the virus needed to infect the apical meristem or germ cells so that the gene editing produced could be stably inherited by the offspring. Based on these results, it was demonstrated that the gRNA(s) released from the virus genome by Csy4 nuclease worked effectively in single and multiple targets in Cas9-mediated genome editing in N. benthamiana cells using the TRV delivery system. Nucleotide replacement could also be conducted using this approach.
Taken together, this study demonstrates the application of Csy4 nuclease for releasing gRNA from a viral delivery system for Cas9-mediated genome editing. The gRNA and the C4 site sequence were inserted in the MCS under a subgenomic promoter in the TRV vector, without any modifications. Additionally, the vector allowed for easy cloning of multiple gRNAs in tandem for targeting multiple sites. The Csy4-RNA processing system offers a feasible and alternative approach in virus delivery research.
Data Availability
The data that supports the findings of this study are available in the supplementary material of this article or from the corresponding author upon reasonable request.
References
Ai P, Xue D, Wang Y, Zeng S (2023) An efficient improved CRISPR mediated gene function analysis system established in Lycium ruthenicum Murr. Ind Crops Prod 192:116142
Ali Z, Abul-Faraj A, Li L, Ghosh N, Piatek M, Mahjoub A, Aouida M, Piatek A, Baltes NJ, Voytas DF (2015) Efficient virus-mediated genome editing in plants using the CRISPR/Cas9 system. Mol Plant 8:1288–1291
Baltes NJ, Gil-Humanes J, Cermak T, Atkins PA, Voytas DF (2014) DNA replicons for plant genome engineering. Plant Cell 26:151–163
Čermák T, Curtin SJ, Gil-Humanes J, Čegan R, Kono TJ, Konečná E, Belanto JJ, Starker CG, Mathre JW, Greenstein RL (2017) A multipurpose toolkit to enable advanced genome engineering in plants. Plant Cell 29:1196–1217
Cheuk A, Houde M (2018) A new barley stripe mosaic virus allows large protein overexpression for rapid function analysis. Plant Physiol 176(3):1919–1931
Cody W, Scholthof HB, Mirkov TE (2017) Multiplexed gene editing and protein over-expression using a tobacco mosaic virus viral vector. Plant Physiol 175(1):23–35
Curtis MD, Grossniklaus U (2003) A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol 133(2):462–469
Fang Y, Cui L, Gu B, Arredondo F, Tyler BM (2017) Efficient genome editing in the oomycete Phytophthora sojae using CRISPR/Cas9. Curr Protoc Microbiol 44:21A.1.1–21A.1.26
Gil-Humanes J, Wang Y, Liang Z, Shan Q, Ozuna CV, Sánchez-León S, Baltes NJ, Starker C, Barro F, Gao C (2017) High-efficiency gene targeting in hexaploid wheat using DNA replicons and CRISPR/Cas9. Plant J 89:1251–1262
Haurwitz RE, Jinek M, Wiedenheft B, Zhou K, Doudna JA (2010) Sequence-and structure-specific RNA processing by a CRISPR endonuclease. Science 329(5997):1355–1358
Karmacharya A, Li D, Leng Y, Shi G, Liu Z, Yang S, Du Y, Dai W, Zhong S (2023) Targeting disease susceptibility genes in wheat through wide hybridization with maize expressing Cas9 and guide RNA. Mol Plant Microbe Interact 36(9):554–557
Lampropoulos A, Sutikovic Z, Wenzl C, Maegele I, Lohmann JU, Forner J (2013) GreenGate-a novel, versatile, and efficient cloning system for plant transgenesis. PLoS ONE 8:e83043
Liu H, Zhang B (2020) Virus-based CRISPR/Cas9 genome editing in plants. Trends Genet 36(11):810–813
Liu Y, Schiff M, Marathe R, Dinesh-Kumar S (2002) Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N-mediated resistance to tobacco mosaic virus. Plant J 30:415–429
Massel K, Lam Y, Hintzsche J, Lester N, Botella JR, Godwin ID (2022) Endogenous U6 promoters improve CRISPR/Cas9 editing efficiencies in Sorghum bicolor and show potential for applications in other cereals. Plant Cell Rep 41(2):489–492
Nekrasov V, Staskawicz B, Weigel D, Jones JD, Kamoun S (2013) Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat Biotechnol 31:691–693
Nissim L, Perli SD, Fridkin A, Perez-Pinera P, Lu TK (2014) Multiplexed and programmable regulation of gene networks with an integrated RNA and CRISPR/Cas toolkit in human cells. Mol Cell 54(4):698–710
Oh Y, Kim H, Lee HJ, Kim SG (2021) Ribozyme-processed guide RNA enhances virus-mediated plant genome editing. Biotechnol J e2100189
Polstein LR, Gersbach CA (2015) A light-inducible CRISPR-Cas9 system for control of endogenous gene activation. Nat Chem Biol 11(3):198–200
Shan Q, Wang Y, Li J, Gao C (2014) Genome editing in rice and wheat using the CRISPR/Cas system. Nat Protoc 9:2395–2410
Tang X, Zheng X, Qi Y, Zhang D, Cheng Y, Tang A, Voytas DF, Zhang Y (2016) A single transcript CRISPR-Cas9 system for efficient genome editing in plants. Mol Plant 9(7):1088–1091
Terry LJ, Shows EB, Wente SR (2007) Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science 318:1412–1416
Velásquez AC, Chakravarthy S, Martin GB (2009) Virus-induced gene silencing (VIGS) in Nicotiana benthamiana and tomato. J Vis Exp 28:1292
Wang M, Lu Y, Botella JR, Mao Y, Hua K, Zhu J-K (2017) Gene targeting by homology-directed repair in rice using a geminivirus-based CRISPR/Cas9 system. Mol Plant 10(7):1007–1010
Wang JY, Doudna JA (2023) CRISPR technology: a decade of genome editing is only the beginning. Science 379(6629):eadd8643
Xie K, Yang Y (2019) A multiplexed CRISPR/Cas9 editing system based on the endogenous tRNA processing. Methods Mol Biol 1917:63–73
Xing H-L, Dong L, Wang Z-P, Zhang H-Y, Han C-Y, Liu B, Wang X-C, Chen Q-J (2014) A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol 14:327
Yin K, Han T, Liu G, Chen T, Wang Y, Yu AYL, Liu Y (2015) A geminivirus-based guide RNA delivery system for CRISPR/Cas9 mediated plant genome editing. Sci Rep 5:14926
Zetsche B, Volz SE, Zhang F (2015) A split-Cas9 architecture for inducible genome editing and transcription modulation. Nat Biotechnol 33(2):139–142
Zhang Y, Liang Z, Zong Y, Wang Y, Liu J, Chen K, Qiu J-L, Gao C (2016) Efficient and transgene-free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nat Commun 7:12617
Acknowledgements
We thank Addgene for providing plasmids PGK1p-Csy4-Pa, pGGB003, pGGZ001, and pHSN6A01. We are also grateful to Dr. Ruyi Xiong and Dr. Fangfang Li for their helpful discussions.
Funding
This work was supported by Scientific Research Start-up Fund Project for the introduction of high-level talents of Baotou Teacher’s College (BTTCRCQD2023-003, BTTCRCQD2023-004), Hebei Province Funding Project for the Introduction of Overseas Students (C20210509), Hebei Natural Science Foundation (C2020301040, C2019301077), and National Natural Science Foundation of China (31400266, 31501625), Agri-Food Canada (AAFC).
Author information
Authors and Affiliations
Contributions
Ren Na and Lining Tian formulated and planned the research. Ren Na and Yanjie Luo designed experiments; Yanjie Luo, Ren Na, Xiaodong Tang, Cuihong Yu, Yang Qiu, Julia S. Nowak, and Qing Shi Lu performed the experiments; Yanjie Luo and Ren Na analyzed the data and wrote the manuscript. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Message
• A plant virus-based gRNA delivery system is a promising method to facilitate the application of CRISPR/Cas9 in genome editing. In this study, we used the Csy4-RNA processing system to release gRNA(s) from a plant virus-TRV vector. We showed that the Csy4-processed TRV-based delivery system was effective in targeting single and multiple sites in the Cas9-mediated genome editing. Our results suggest that this system is a useful tool for genome engineering in plants.
Supplementary Information
Below is the link to the electronic supplementary material.
11105_2024_1459_MOESM1_ESM.docx
Additional supporting information may be found online in the Supporting Information section at the end of the article: Fig. S1. Primers used in the project. Fig. S2. Off-target analysis. Fig. S3. MCS site sequences of pTRV2 derivatives. Fig. S4. The sequence of Cas9 and Csy4 inserted into pGGZ001. Fig. S5. Donor template sequence. (DOCX 1041 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luo, Y., Tang, X., Yu, C. et al. Development of a Csy4-Processing TRV-Based CRISPR/Cas9 Genome Editing System in Nicotiana benthamiana. Plant Mol Biol Rep (2024). https://doi.org/10.1007/s11105-024-01459-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11105-024-01459-0