Abstract
Purpose
The ecological cultivation of Panax notoginseng under a forest canopy relies on the coupling of the P. notoginseng growth environment and the forest ecosystem
Methods
In this study, six tree species, such as Platycladus orientalis (L.) Franco, were chosen to research the effects of species interactions on the growth, quality, and disease occurrence under intercropping with P. notoginseng, with single P. notoginseng serving as the control.
Results
Intercropping P. notoginseng with PO (Platycladus orientalis, a coniferous tree species) or with SW (Schima wallichii Choisy, a broad-leaved tree species) promoted the accumulation of P. notoginseng biomass, reduced the occurrence of root rot, improved the contents of nitrogen, phosphorus and potassium in P. notoginseng, and increased the saponin concentration. Then, 43 differentially abundant metabolites were screened in the P. notoginseng-tree intercropping system by soil metabolism analysis and compared with those in the monocropped system. Indole-3-carboxaldehyde showed a significant negative relationship with the occurrence of root rot disease and inhibited Fusarium oxysporum. In addition, 2-naphthalenesulfonic acid was significantly positively correlated with biomass and increased the dry weight in the underground part of P. notoginseng in the pot experiments.
Conclusions
Thus, the coniferous tree species PO and the broad-leaved tree species SW are potentially good neighbours of P. notoginseng, and soil metabolic changes may be important mechanisms for the growth and disease resistance benefits observed in the understorey of P. notoginseng.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intercropping is a typical model of traditional agriculture and is a fundamental and prevalent practice in modern ecological cultivation (Zhang et al. 2023a and 2023b; Wu and Lin 2020). The intercropping system leverages the principle of species diversity to foster beneficial interactions between different plant species (Chen 2023; Peng, et al. 2020; Wen, et al. 2023a, 2022), resulting in notable productivity advantages. These benefits include increased yield (Finn et al. 2013; Guay et al. 2018; Li et al. 2020a, b), improved crop quality (Bélanger et al. 2014), reduced impacts from pests and diseases (Boudreau 2013; Gaba et al. 2015), soil conservation (Weyers et al. 2021), and carbon sequestration (Malézieux et al. 2009). Agroforestry, as a form of intercropping system, utilizes abundant forest resources and the ecological environment beneath the forest canopy, thus offering great potential for cultivating shade-loving plants. Chinese medicinal herbs such as P. notoginseng (Wang et al. 2021a; Xiong et al. 2022; Zhu et al. 2022), Polygonatum kingianum (Wang 2023) and Bletilla striata (Wan et al. 2018; Deng et al. 2023) are highly suitable for agroforestry intercropping under a forest canopy due to their extended cultivation period and compatibility with the native environment, additionally, these plants have significant economic value.
An innovative intercropping system, the cultivation of P. notoginseng in forests, has been developed and extensively tested in southwestern China. The implementation of this system has the potential to yield substantial social, economic, and environmental benefits (Ye et al. 2019). Moreover, this system can effectively facilitate the growth and enhance the quality of medicinal plants while concurrently mitigating pests and disease infestations (Wang et al. 2021a, b; Xiong et al. 2022). For instance, the quality of P. notoginseng divaricate cultivated in pine forests was slightly better than that cultivated in the field especially the content of saponins (Jia et al. 2022; Hei et al. 2023). The saponins were a critical component of P. notoginseng, exhibiting diverse pharmacological effects and health-promoting properties. Furthermore, they served as a reliable indicator of the overall quality of P. notoginseng. The key medicinal constituent of P. notoginseng as saponins, specifically including Notoginsenoside R1, Ginsenoside Rb1, Ginsenoside Rd, Ginsenoside Re, and Ginsenoside Rg1 etc. (Chen et al. 2014; Liu et al. 2017). The forest environment provides optimal conditions for the growth of P. notoginseng, including suitable shade, appropriate temperature and moisture levels, as well as an abundance of essential nutrients, all of which contribute to its thriving development (Ye et al. 2019). The release of allelochemicals, including volatile organic compounds and leachate, by pine trees plays a pivotal role in enhancing the disease resistance of P. notoginseng (Ye et al. 2021; Li et al. 2023). However, there is currently no available information regarding direct root interactions between trees and P. notoginseng under field conditions.
Interspecific root interactions between intercropping species play pivotal roles in augmenting nutrient absorption and regulating soil-borne disease occurrence in agroforestry systems (Deng et al. 2023; Yang et al. 2022; Zhou et al. 2023a, b). Studies have reported that, compared with pepper monocultures, intercropped maize plants grown between pepper rows have reduced disease levels of pepper Phytophthora blight, which is related to the ability of maize plants to form a ‘root wall’ that restricts the movement of Phytophthora capsici across rows (Yang et al. 2014). Intercropping systems promote the development of a disease-suppressive rhizosphere microbiome, which confers protection to tomato plants against Verticillium wilt disease caused by the soil-borne pathogen Verticillium dahliae (Zhou et al. 2023b, a). The cultivation of P. notoginseng also faces significant challenges associated with root rot, which can result in compromised growth, disease susceptibility, diminished quality, reduced yield, or even complete crop failure (Cui et al. 2014; Yang et al. 2018). Therefore, assessing the interspecific interactions between P. notoginseng roots and trees is highly useful in agroforestry systems.
Root exudates play a pivotal role in mediating species-specific rhizosphere interactions, encompassing signal transmission, the activation of soil nutrient reservoirs, the modulation of rhizosphere microbial communities, and the induction of plant resistance (Hu et al. 2021; Li et al. 2020b; Dong et al. 2021). Numerous studies have demonstrated that various natural antimicrobial substances can be secreted by plant roots, creating defence networks in the rhizosphere (Zhu et al. 2021). This mechanism not only inhibits soil-borne pathogens in a sustainable manner but also prevents the development of drug resistance. Phenolic acids, terpenes and isothiocyanate secreted by nonhost plant roots significantly reduced Phytophthora blight in pepper and soybean plants (Zhang et al. 2019a, b; Li et al. 2022a, b). The intercropping of Platycodon grandiflorum and Welsh onion increased the content of benzothiazole and 2-methylthiobenzothiazole in the root exudates of P. grandiflorum and alleviated the continuous cropping obstacle of P. grandiflorum caused by F. pythium (Wang et al. 2019). Intercropping watermelon with wheat increased the release of coumaric acid in wheat roots and inhibited the growth of the watermelon pathogenic fungus F. oxysporum (Lv et al. 2018). Besides, as plant rhizosphere secretions, indole played an important role in regulating plant growth in intercropping systems (Xiao 2023). Indole was an aromatic heterocyclic compound, and its structural skeleton is widely found in many plants, such as jasmine, orange, daffodils, vanilla and robinia (Millan et al. 2022). Indole-3-carboxaldehyde (I3A) was found in a variety of plants and has been shown to have several biological effects, including effects on plant growth and development (Teng et al. 2018). Recent studies also suggested that I3A was involved in plant metabolism (Ge et al. 2024).
This study focused on the ‘P. notoginseng-tree species’ intercropping mode. Hence, from the viewpoint of the interaction between trees and the rhizosphere of P. notoginseng, the present study delves into the influence and the underlying mechanisms that foster the enhanced growth, disease resistance, and quality augmentation of P. notoginseng. Overall, we aimed to address the following: (1) To investigate the effects of intercropping P. notoginseng with different tree species on growth, quality, and disease incidence; (2) to investigate the impact of intercropping P. notoginseng with various tree species in agroforestry on the rhizosphere soil metabolism of P. notoginseng using liquid chromatography-tandem mass spectrometry (LC–MS/MS); and (3) to verify the inhibitory effects of key compounds in P. notoginseng rhizosphere metabolites on root rot pathogens and their ability to promote the growth of P. notoginseng. Based on the above research, we hoped to find the key metabolites in the root interaction between trees and P. notoginseng, and thus select the suitable tree species to match with P. notoginseng. The purpose of this study was to provide technical support for the selection of tree species for planting P. notoginseng in forestland and to provide practical recommendations for the biomimetic cultivation of P. notoginseng within forests.
Materials and methods
Experimental design and growth conditions
Experiments were conducted at the experimental station of Yunnan Agricultural University in Xundian County, Yunnan, China (103.286°E, 25.521°N; altitude of 1960 m). Seven different systems were used, namely, P. notoginseng monocropping (CK) and P. notoginseng respectively intercropped with Eucalyptus robusta Smith (ER), Cunninghamia lanceolata (CL), Platycladus orientalis (PO), Schima wallichii Choisy (SW), Alnus cremastogyne (AN), and Pistacia weinmannifolia J. Poisson ex Franch (PW). The reason for selecting these six tree species for experimentation was carefully considered as: firstly, these six tree species have large planting areas and widespread distribution in Yunnan Province, China, rendering them highly representative. Secondly, the spacing of trees in these forests is suitable for understory planting and field management. Additionally, the canopy architecture of these trees is conducive to intercepting appropriate sunlight, providing suitable shading for the healthy growth of P. notoginseng under the forest canopy. In this sense, six different trees with heights between 1.0 and 1.5 m were selected and planted in a greenhouse. Then, 15 P. notoginseng plants were planted under these trees 10 cm from the trunks of the trees (Fig. 1). All pots were arranged according to a completely randomized block design. The greenhouse was shaded with a polyethylene net that allowed 10% light transmission to simulate the natural conditions for P. notoginseng growth (Chen et al. 2014). The temperature was controlled at 18–30 °C. Throughout the experiment, consistent field management measures such as water and fertilizer management and pest control were implemented by intercropping modes.
Pot experiment diagram, of the intercropping modes (A) and the P. notoginseng monoculture (B). Legend: In Fig. 1A, the arrowed straight line illustrates the direct distance between P. notoginseng and the tree species, while the circle denotes the soil sampling position of the interacting soil. At this position, the roots of P. notoginseng and the tree species have established an interactive relationship. In Fig. B, the circles represent the single-plot soil sample collection location, and the arrow distance indicates the position between Panax notoginseng, consistent with that in Fig. A. The circle signifies the sampling point of P. notoginseng, aligning with its position in Fig. 1A
Measuring the growth and disease status of P. notoginseng under different intercropping systems
To evaluate the growth of P. notoginseng in the seven intercropping systems, the germination and survival rates of P. notoginseng were investigated in April and November, respectively, and calculated as follows:
To investigate the impact of intercropping P. notoginseng with different tree species on plant health, plants that displayed symptoms of root rot were recorded. Once the P. notoginseng plants were harvested, the soil and debris on the root surface were rinsed off. The remaining soil attached to the roots was gently brushed away with a soft bristle brush. Subsequently, the incidence of root disease in P. notoginseng was calculated, and the severity of the root disease was assessed by classifying and quantifying the disease based on the severity of the disease (Table 1).
In August, P. notoginseng was collected and brought to the laboratory after the final stored seedlings were counted. The general samples were washed to remove the soil, dried to remove surface moisture, and weighed (fresh weight); then, the plants were dried to a constant weight at 60 °C in an oven, cooled to 20 °C, and weighed again (dry weight). Sixty plants were tested in each treatment and divided into three replicates; each replicate contained 20 plants. The average weight of a single plant in each replicate was subsequently calculated. Finally, the yield per unit area and root-to-shoot ratio were calculated using the following formulas:
Effect of seven intercropping modes on the nutritional status of P. notoginseng plants
The aboveground components and underground roots of the oven-dried plants were digested in a mixture of H2SO4 and H2O2, and the N, P, and K concentrations in the dry matter of the aboveground components and underground roots were measured via the micro-Kjeldahl procedure, vanadomolybdate method, and flame photometry, respectively (Du et al. 2024; Lu 2000).
Analysis of the saponin content
The extraction of saponins was conducted as previously described (Liu et al. 2021). The detailed process was as follows: firstly, the oven-dried roots of P. notoginseng were grinded into powder and sift through a 250-µm mesh sieve for uniform mixing. Then a total of 0.2 g of P. notoginseng root powder was accurately weighed, placed in a conical flask with a grinding mouth, added with 15 mL of 75% methanol, ultrasonically extracted at 40 °C for 40 min, taken out and allowed to stand for 40 min. After filtration, the filtrate was filtered with a microporous membrane (0.45 μm), and the content was determined by HPLC (Yang et al. 2022). The target compounds in minimal medium were identified by comparing their retention times to those of ginsenoside standards Ginsenoside Re (CAS number 51542–56-4), Ginsenoside Rg1(CAS number 22427–39-0), Ginsenoside Rd (CAS number 52705–93-8), Ginsenoside Rb1 (CAS number 41753–43-9), and Notoginsenoside R1 (CAS number 80418–24-2), which were purchased from Guizhou Dida Biological Technology Co (Guizhou, China). The content of the target compounds in minimal medium was quantified using standard curves that showed the linear relationships between the peak areas and the concentrations (Luo et al. 2021). Analysis were performed using an UHPLC (1290 Infinity LC, Agilent Technologies) coupled to a quadrupole time-of-flight (AB Sciex Triple TOF 6600) in Shanghai Applied Protein Technology Co., Ltd. A chromatographic column (Waters, Ireland, 1.8 μm, C18, 100 A, 100 mm × 2.1 mm) was used. The initial injection volume was 2 μL, and the flow rate was 0.6 mL min−1. The column temperature was maintained at 40 °C, and the detection wavelength was 203 nm. The mobile phase was a linear gradient elution of water (A)-acetonitrile (B) (V/V). The levels of R1, Rg1, Re, Rb1, and Rd saponins in the roots were measured using a Nexera X2 UPLC system (Shimadzu, Japan). The instrument parameters were set according to the methods of Liu (Liu et al. 2021).
Collection of soil samples and detection of soil physicochemical properties
Bulk soil samples were collected at locations where the root systems of P. notoginseng and the intercropping trees were intertwined. The samples were placed in an icebox and taken back to the laboratory, after which the soil was separated into two parts. One was packed in 2 ml centrifuge tubes and stored in a liquid nitrogen tank until frozen. The plants were then transported back to the laboratory for soil metabolite detection. The second subsample was dried and sieved for the determination of potential of hydrogen (pH) and electrical conductivity (EC). The soil pH and EC were determined using an Inolab pH/Cond 720 in a water suspension (water:soil, 2.5:1, v:w) following the method described by Abdalmoula (Abdalmoula et al. 2019).
Soil metabolite extraction and analysis
Analysis was performed by using an UHPLC (1290 Infinity LC, Agilent Technologies) instrument equipped with a 2.1 mm × 100 mm ACQUIY UPLC BEH 1.7 μm column (Waters, Ireland) coupled to a quadrupole time-of-flight (AB Sciex Triple TOF 6600). The column oven was maintained at 25 °C, and the flow rate was 0.5 mL/min. In both the ESI-positive and ESI-negative modes, the mobile phase contained 25 mmol/L ammonium acetate and 25 mmol/L ammonium hydroxide in water (A) and acetonitrile (B). The gradient was 95% B for 0.5 min, linearly reduced to 65% in 7 min, decreased to 40% in 1 min, maintained for 1 min, and subsequently increased to 95% in 0.1 min, with a 3 min re-equilibration period. The MS experiment was performed with the ESI source under the following conditions: Ion Source Gas1 (Gas1) and Ion Source Gas2 (Gas2) were both set to 60 psi, the curtain gas (CUR) pressure was 30 psi, the source temperature was 600 ◦C, and the ion spray voltage floating (ISVF) was ± 5500 V. In MS-only acquisition, the mass range of the instrument was from m/z 60 to 1000 Da, and the accumulation time for the TOF MS scan was set at 0.20 s/spectra. Moreover, for auto MS/MS acquisition, the mass range was set to 25–1000 Da with 0.05 s/spectra of the accumulation time for the production scan. The product ion scan was acquired using information-dependent acquisition (IDA) with high-sensitivity mode selected. The parameters were set as follows: the collision energy (CE) was fixed at 35 V ± 15 eV; the declustering potentials (DPs) were 60 V ( +) and − 60 V ( −); and the number of isotopes within 4 Da and candidate ions were excluded for monitoring every cycle: 10. Refer to Chen's method for compound identification (Yang et al. 2023; Chen et al. 2022).
Effects of indole-3-carboxaldehyde on the growth of Fusarium
The effects of indole-3-carboxaldehyde on the growth of culturable Fusarium were measured following a published procedure with some modifications (Lv et al. 2018). Briefly, a mycelium block of Fusarium solani (main pathogen of root rot disease of P. notoginseng, isolated from the rot root of P. notoginseng and identified by ITS sequencing and Coch's rule, as detailed in Fig. S1, 6 mm diameter) was placed in the middle of Potato dextrose agar medium (PDA) media supplemented with indole-3-carboxaldehyde (at final concentrations of 0, 25, 50, 100, 200, and 400 mg/L). Six replicate plates were used for each concentration. The mycelial growth of the isolates was determined by measuring the colony semidiameter after dark incubation at 25 °C for 5 days. The growth inhibition rate was calculated as follows:
Effects of soil metabolites on plant biomass
To test the effect of indole-3-carboxaldehyde on plant growth, we evaluated the effect of plant biomass on P. notoginseng after irrigation with indole-3-carboxaldehyde. The P. notoginseng seeds were immersed in 1% sodium hypochlorite for 5 min and then washed three times with sterile water. Seeds were sown in a pot (15.5 cm × 11.5 cm × 13.5 cm) and cultivated for half a year for growth analysis. All the pots were placed in a greenhouse shaded with a polyethylene net that allowed 10% full sunlight transmission. After six months, the plants in all the pots were subjected to the growth-promoting effects of indole-3-carboxaldehyde. Fifty millilitres of indole-3-carboxaldehyde (at final concentrations of 0, 1, 3, and 5 mg/L) was added to the soil via irrigation once a week. Each treatment included six pots. Then, 2 months after treatment, the plant weights were recorded.
Statistical analyses
IBM SPSS Statistics version 27 (SPSS, Inc., Chicago, Illinois, USA) was used for general statistical analyses. One-way analysis of variance (ANOVA) and Turkey HSD corrections (p < 0.05) were used to analyse the mean separations among treatments. Box plots were drawn with GraphPad Prism 10 (GraphPad Software, San Diego, CA, USA). Referring to Li's method, we performed soil metabolite analysis after appropriate improvement (Li and Jiang 2021). Partial least squares discriminant analysis (PLS-DA) was conducted to analyze and verify the differences and reliability of metabolites in the samples. The metabolite content data were normalized by the range method, and the accumulation mode of metabolites among different samples was analyzed by heatmap cluster analysis (hierarchical cluster analysis, HCA) through using the OmicStudio tool (https://www.omicstudio.cn/tool). PLS-DA analysis was applied to calculate the corresponding variable importance in projection (VIP) value. PLS-DA, volcano plot and univariate statistical analysis t-test were used to screen the differential metabolites between groups. Metabolites with Fold Change > 2 or Fold Change < 0.5, VIP ≥ 1 and P ≤ 0.5 were considered differentially expressed metabolite. Then, spearman correlation analysis was used to comprehensively analyzed the differential metabolites and field indicators to further determine the differential metabolites affecting the disease and growth of P. notoginseng.
Results
Effects of different intercropping modes on the growth and disease status of P. notoginseng
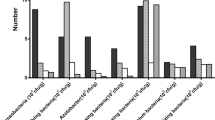
The seeding germination and survival rates of P. notoginseng were affected when P. notoginseng was intercropped with different tree species (Fig. 2A and B). The germination rates of P. notoginseng significantly increased when cultivated under PO (61.89%), CL (68.89%) and ER (69.49%) but decreased under PW (39.72%) (Fig. 2A). After intercropping, P. notoginseng cultivated under SW (34.07%), CL (45.31%), PO (42.89%), and ER (41.21%) had significantly greater seedling survival rates than that cultivated under the control treatment (20.32%) (Fig. 2B). Interestingly, when P. notoginseng was planted with different tree species, the incidence of root rot was significantly lower (Fig. 2C). Furthermore, disease indices showed that, in comparison to the control treatment, all the intercropping modes, except for AN, significantly lowered the incidence of root rot disease in P. notoginseng, especially PO (14.01%), CL (11.61%), SW (18.81%), PW (22.33%) and ER (12.09) (Fig. 2D). These results indicate that the interactions of P. notoginseng with PO, CL and ER were more conducive to seedling growth and reduced root rot disease incidence.
Effects of seven intercropping systems on the growth and disease status of P. notoginseng. A. Germination rates (F = 33.524, Degrees of Freedom = 6, p < 0.01). B Seedling survival rates (F = 15.989, Degrees of Freedom = 6, p < 0.01). C Incidence rate of P. notoginseng root rot disease (F = 139.137, Degrees of Freedom = 6, p < 0.01). D Indices of P. notoginseng root rot disease (F = 25.602, Degrees of Freedom = 6, p < 0.01). Each cycle in one box represents a biologically independent sample. Horizontal bars within boxes represent the median. The tops and bottoms of boxes represent 75th and 25th quartiles, respectively. Different lowercase letters indicated significant differences among respective groups based on two-sided tests for multiple comparisons by Turkey HSD corrections (ANOVA, adjusted p < 0.05, n = 6 biologically independent samples).Legend: CK: P. notoginseng monocropping; ER: P. notoginseng intercropped with Eucalyptus robusta Smith; CL: P. notoginseng intercropped with Cunninghamia lanceolata; PO: P. notoginseng intercropped with Platycladus orientalis; SW: P. notoginseng intercropped with Schima wallichii Choisy; AN: P. notoginseng intercropped with Alnus cremastogyne; PW: P. notoginseng intercropped with Pistacia weinmannifolia J. Poisson ex Franch
Appropriate effects of intercropping modes were more conducive to increasing P. notoginseng biomass
To evaluate the real growth of P. notoginseng under the seven intercropping patterns, the yield per square metre was calculated according to the dry weight per plant and the seedling survival rate. In this study, the biomass of P. notoginseng was influenced when it was cultivated under the seven intercropping modes (Table 2). The dry weight per unit area of aboveground of P. notoginseng was significantly greater in the PO and CL treatments than in the CK treatment. The underground dry weight per unit area of P. notoginseng was significantly greater in the PO, CL, SW, and ER treatments than in the CK treatment. A comparison of the root-to-shoot ratio of P. notoginseng revealed that the PW treatment had a significantly lower ratio than the CK treatment. Taken together, these results indicate that the intercropping of P. notoginseng with PO and CL resulted in greater aboveground dry weight and underground dry weight than did the monocropping of P. notoginseng, as well as, the underground dry weight of P. notoginseng of SW and ER were notably elevated compared to the control.
Effect of seven intercropping modes on the nutritional status of P. notoginseng
Significant differences in plant nutrition were observed among the seven intercropping systems. The total nitrogen content of aboveground of P. notoginseng in CL was significantly greater than that of CK (Fig. 3A); the underground total nitrogen content of P. notoginseng in PO and CL was significantly greater than that of CK (Fig. 3D); the aboveground total phosphorus in PO and CL was significantly greater than that of CK (Fig. 3B); the underground total phosphorus in PO, CL and SW was significantly greater than that of CK (Fig. 3E); and POP and SW were significantly greater in total potassium aboveground than CK (Fig. 3C). Similarly, the underground parts of CL, AN and ER contained significantly less total potassium than those of CK (Fig. 3F). These results suggested that the intercropping system promoted P. notoginseng nutrient uptake: PO and CL were more conducive to nitrogen uptake, PO was more conducive to aboveground phosphorus uptake and PO, SW and ER were more conducive to aboveground potassium uptake compared with single-cropped P. notoginseng.
Effects on characteristics of N, P, and K in P. notoginseng of different intercropping modes. A Aboveground total nitrogen (F = 10.617, Degrees of Freedom = 6, p < 0.01). B Aboveground total phosphorus (F = 84.063, Degrees of Freedom = 6, p < 0.01). C Aboveground total potassium (F = 28.804, Degrees of Freedom = 6, p < 0.01). D Underground total nitrogen (F = 18.202, Degrees of Freedom = 6, p < 0.01). E Underground total phosphorus (F = 96.526, Degrees of Freedom = 6, p < 0.01). F Underground total potassium (F = 14.561, Degrees of Freedom = 6, p < 0.01). Each cycle represents a biologically independent sample. Horizontal bars within boxes represent the median. The tops and bottoms of boxes represent 75th and 25th quartiles, respectively. Different lowercase letters indicated significant differences among respective groups based on two-sided tests for multiple comparisons by Turkey HSD corrections (ANOVA, adjusted p < 0.05, n = 6 biologically independent samples). Legend: CK: P. notoginseng monocropping; ER: P. notoginseng intercropped with Eucalyptus robusta Smith; CL: P. notoginseng intercropped with Cunninghamia lanceolata; PO: P. notoginseng intercropped with Platycladus orientalis; SW: P. notoginseng intercropped with Schima wallichii Choisy; AN: P. notoginseng intercropped with Alnus cremastogyne; PW: P. notoginseng intercropped with Pistacia weinmannifolia J. Poisson ex Franch
Effect of seven intercropping modes on the saponin content of P. notoginseng
The saponins in the seven intercropping systems of P. notoginseng were determined. Initially, the amount of R1 saponins in the PO, CL, SW and PW intercropping systems was significantly greater than that in the CK treatment (Fig. 4A). Second, the amount of the Rg1 saponin in the PO, CL, SW, and PW intercropping systems was significantly greater than that in the CK treatment (Fig. 4B). In addition, the Re saponin content in the PO intercropping treatment was significantly greater than that in the CK treatment (Fig. 4C). Moreover, the abundance of the Rb1 saponin in the PO, SW, and PW intercropping systems was significantly greater than that in the CK treatment (Fig. 4D). Moreover, the Rd saponin content in the PO, SW and PW intercropping systems was significantly greater than that in the CK treatment (Fig. 4E). Finally, the total ginsenoside saponin content in the PO, SW and PW intercropping systems was significantly greater than that in the CK treatment (Fig. 4F). These results indicate that the intercropped P. notoginseng plants under different trees had greater amounts of root saponins than did the monocropped P. notoginseng plants, especially R1, Rg1, Rb1, Rd and the five total ginsenosides in PO, SW and PW intercropping modes.
Effect of seven treatments on the saponins content of P. notoginseng roots. A Notoginsenoside R1 (F = 321.607, Degrees of Freedom = 6, p < 0.01). B Ginsenoside Rg1 (F = 168.925, Degrees of Freedom = 6, p < 0.01). C Ginsenosides Re (F = 28.182, Degrees of Freedom = 6, p < 0.01). D Ginsenoside Rb1 (F = 294.439, Degrees of Freedom = 6, p < 0.01). E Ginsenosides Rd (F = 85.595, Degrees of Freedom = 6, p < 0.01). F Total ginsenosides(R1 + Rg1 + Re + Rb1 + Rd) (F = 272.983, Degrees of Freedom = 6, p < 0.01). Each cycle represents a biologically independent sample. Horizontal bars within boxes represent the median. The tops and bottoms of boxes represent 75th and 25th quartiles, respectively. Different lowercase letters indicated significant differences among respective groups based on two-sided tests for multiple comparisons by Turkey HSD corrections (ANOVA, adjusted p < 0.05, n = 6 biologically independent samples).Legend: CK: P. notoginseng monocropping; ER: P. notoginseng intercropped with Eucalyptus robusta Smith; CL: P. notoginseng intercropped with Cunninghamia lanceolata; PO: P. notoginseng intercropped with Platycladus orientalis; SW: P. notoginseng intercropped with Schima wallichii Choisy; AN: P. notoginseng intercropped with Alnus cremastogyne; PW: P. notoginseng intercropped with Pistacia weinmannifolia J. Poisson ex Franch
Effect of seven intercropping modes on the pH and EC of soil in the interaction regions
pH and EC were selected as indicators of changes in soil chemical properties, therefore, the pH and EC were measured in the seven intercropping systems and showed significant differences between the intercropped and CK soils (Fig. 5). The EC of soil in the interaction regions in the PO, CL, SW and ER treatments was significantly greater than that in the CK treatment (Fig. 5A). The pH values of soil in the interaction regions in SW, CL, PO, AN, and ER were significantly lower than that of CK (Fig. 5B). Taken together, these findings show that the root exudates of trees may change the physical and chemical properties of soils in different intercropping systems.
The pH and EC analysis of soil in the interaction regions of different intercropping modes. A EC of soils (F = 285.711, Degrees of Freedom = 6, p < 0.01). B The pH of soils (F = 10.213, Degrees of Freedom = 6, p < 0.01). Each cycle represents a biologically independent sample. Horizontal bars within boxes represent the median. The tops and bottoms of boxes represent 75th and 25th quartiles, respectively. Different lowercase letters indicated significant differences among respective groups based on two-sided tests for multiple comparisons by Turkey HSD corrections (ANOVA, adjusted p < 0.05, n = 6 biologically independent samples). Legend: CK: P. notoginseng monocropping; ER: P. notoginseng intercropped with Eucalyptus robusta Smith; CL: P. notoginseng intercropped with Cunninghamia lanceolata; PO: P. notoginseng intercropped with Platycladus orientalis; SW: P. notoginseng intercropped with Schima wallichii Choisy; AN: P. notoginseng intercropped with Alnus cremastogyne; PW: P. notoginseng intercropped with Pistacia weinmannifolia J. Poisson ex Franch
Effect of seven intercropping modes on the soil metabolites of P. notoginseng
Soil metabolomics can correlate fingerprint type to environmental factors, soil nutrients, microbial diversity, and plant phenotypes. Thus, we performed a soil metabolomics study of the seven intercropping systems to explore root-mediated underground communication differences in soil metabolites under the different intercropping systems. A total of 630 metabolites, including amino acids, organic acids, carbohydrates, ketones, lipids, alcohols, etc., were detected and identified among all the soil samples. Lipids and lipid-like molecules were the most common compounds detected, accounting for 38% of all the metabolites, followed by organic acids and their derivatives (24%) and benzenoids (20%) (Fig. S2). The PLS-DA analysis demonstrated that the metabolite profiles associated with the interaction of the six species differed significantly from those associated with the control group (Fig. 6A). To reveal the accumulation patterns of metabolites in the trees and CK, pairwise comparisons of PO vs CK, CL vs CK, SW vs CK, PW vs CK, AN vs CK, and ER vs CK were conducted. 10 significant metabolites were identified between PO and CK (6 increased and 4 decreased). In addition,10 significant metabolites were identified in CL vs CK (1 increased and 9 decreased), and 17 significant metabolites were identified in SW vs CK (1 increased and 16 decreased). 35 significant metabolites were identified between PW and CK (28 increased and 7 decreased). In addition, 22 significant metabolites were identified in AN vs CK (20 increased and 2 decreased), and 29 significant metabolites were identified in ER vs CK (12 increased and 17 decreased) (Fig. 6B). Overall, there were 77 metabolites, with 41 upregulated and 36 downregulated. Based on the results of FC > 2 or FC < 0.5, VIP > 1 and p-value < 0.05, 43 metabolites (23 increased and 20 decreased in CK) were then selected to validate our hypothesis that the soil metabolites assemble a metabolome that leads to promoting healthy growth of P. notoginseng (Fig. S3). The selected metabolites was increased in six intercropping modes including eplerenone hydroxy acid, thalsimine, zinniol, MG (18:2(9Z,12Z)/0:0/0:0) (rac), 1-palmitoylglycerol, octadecanoic acid, 1-stearoyl-sn-glycerol, 2-carboxybenzaldehyde, indole-3-carboxaldehyde, diethylene glycol, 2-deoxy-d-glucose, 4-imidazoleacetic acid, imidazole, 3-hydroxy-3',4',5'-trimethoxyflavone, imidazoleacetic acid, L-glutamic acid, thymidine, 5'-monophosphate, 4-hydroxybenzaldehyde, 3',5'-cyclic inosine monophosphate, ethylenediaminetetraacetic acid, cis,cis-muconic acid, 2,6-di-tert-butyl-4-methoxyphenol and ginsenoside Rf, while those significant decreased in intercropping modes including 2,4,6-tri-tert-butylaniline, spermidine, (r)-( +)-arachidonyl-1'-hydroxy-2'-propylamide, N-palmitoyl-d-sphingosine, N6-methyl-L-lysine, 2-naphthalenesulfonic acid, linoleic acid, 1-o-(9z-octadecenyl)-sn-glycero-2,3-cyclic-phosphate, benzenesulfonic acid, cis-9-palmitoleic acid, 1,2-dioleoyl-sn-glycero-3-phosphate, amastatin, gamma-Glu-Cys, andrastin a, 2-methylamino-1-phenylbutane, N-benzylacetamidine, dibutyl phthalate, 3-cyclohexyl-1,1-dimethylurea, valerenic acid and confertifoline (Fig. S3). Among the 43 compounds, 25.58% of Lipids and lipid-like molecules, 18.60% of Benzenoids, 13.95% of Organic acids and derivatives, and 9.30% of Organoheterocyclic compounds were included (Fig. S4).
Soil metabolite analysis of the seven intercropping systems and CK. A PLS-DA of the samples from seven treatments. The x-axis represents the first principal component, and the y-axis represents the second principal component. Each point represents a biologically independent sample in PLS-DA. Among them, R2 represented the predictive ability of the model, and R2 > 0.7 indicates that the model is excellent; Q2 indicated the predictive ability of the model, the absolute value of Q2 was greater than 0.5, and it indicated that the model was stable and reliable. B Volcano plot of differentially abundant metabolite clustering for the six trees vs CK comparison. The volcano plot map reflects the differences in metabolite expression levels in the two samples. The vertical coordinates represent -log10 (p-value), and the horizontal coordinate values are the log2 (fold changes). Each point in the graph represents a detected metabolite. The legend of the volcano plot indicates the number of significant Metabolites. Legend: CK: P. notoginseng monocropping; ER: P. notoginseng intercropped with Eucalyptus robusta Smith; CL: P. notoginseng intercropped with Cunninghamia lanceolata; PO: P. notoginseng intercropped with Platycladus orientalis; SW: P. notoginseng intercropped with Schima wallichii Choisy; AN: P. notoginseng intercropped with Alnus cremastogyne; PW: P. notoginseng intercropped with Pistacia weinmannifolia J. Poisson ex Franch
Inhibition test of metabolites against F. solani
Correlation analysis of 43 selected compounds with the incidence and disease indices of P. notoginseng root rot revealed a significant negative correlation between the indole-3-carboxaldehyde concentration with incidence rate (R = -0.40, p < 0.01) and disease index (R = -0.37, p < 0.05); however, the 2-naphthalenesulfonic acid concentration was significantly positively correlated with the incidence rate R = 0.48, p < 0.01) (Fig. 7A). Therefore, indole-3-carboxaldehyde and 2-naphthalenesulfonic acid were selected for validation. The mycelial growth of F. solani was significantly inhibited by indole-3-carboxaldehyde when the concentration of indole-3-carboxaldehyde reached 25 mg/L (inhibition rate = 3.01, p < 0.05) (Fig. 7B); as the concentration of indole-3-carboxaldehyde increased, the inhibitory ability increased (R = 0.51, p < 0.01) (Fig. 7B). The mycelial growth of F. solani was significantly promoted by 2-naphthalenesulfonic acid when the 2-naphthalenesulfonic acid concentration reached 25 mg/L (inhibition rate = − 1.48, p < 0.05) (Fig. 7D), but the mycelial growth of F. solani was significantly inhibited by 2-naphthalenesulfonic acid when the 2-naphthalenesulfonic acid concentration reached 50 mg/L and the inhibition ability increased (inhibition rate = 3.77, R = 0.51, p < 0.05) (Fig. 7D). Treatment of P. notoginseng with indole-3-carboxaldehyde at 0.1 mg/L significantly increased the survival rate, and the survival rate reached its maximum value at 0.25 mg/L (Fig. 7C). However, there was no significant difference in the survival rate of seedlings treated with 2-naphthalenesulfonic acid compared to that of the control group, but the survival rate showed a downwards trend as the concentration increased (Fig. 7E). Specifically, the content of indole-3-carboxaldehyde in the CK treatment was significantly lower than that in the intercropped tree species, and the content of 2-naphthalenesulfonic acid in the CK treatment was significantly greater than that in the intercropped tree species. These findings are consistent with the incidence of root rot in the field.
Screening and validation of disease-related metabolites. A Heatmap of the correlation between differentially abundant metabolites and the incidence and disease indices of P. notoginseng root rot. Antagonistic test results of indole-3-carboxaldehyde (B) and 2-naphthalenesulfonic acid treatments (D) against Fusarium solani. Different lowercase letters indicated significant differences among respective groups based on two-sided tests for multiple comparisons by Turkey HSD corrections (ANOVA, adjusted p < 0.05, n = 6 biologically independent samples, indole-3-carboxaldehyde treatment: F = 41.126, Degrees of Freedom = 4, p < 0.01; 2-naphthalenesulfonic acid treatment: F = 65.327, Degrees of Freedom = 4, p < 0.01). The survival rate of P. notoginseng seedlings after indole-3-carboxaldehyde treatment (C) and 2-naphthalenesulfonic acid (E). Each cycle represents a biologically independent sample. Horizontal bars within boxes represent the median. The tops and bottoms of boxes represent 75th and 25th quartiles, respectively. Different lowercase letters indicated significant differences among respective groups based on two-sided tests for multiple comparisons by Turkey HSD corrections (ANOVA, adjusted p < 0.05, n = 6 biologically independent samples, indole-3-carboxaldehyde treatment: F = 9.198, Degrees of Freedom = 4, p < 0.01; 2-naphthalenesulfonic acid treatment: F = 1.376, Degrees of Freedom = 4, p > 0.05)
Effects of metabolites on dry matter accumulation in P. notoginseng
Correlation analysis between 43 metabolites and the aboveground and underground dry weights of P. notoginseng revealed significant relationships between metabolites and plant growth. Specifically, 2-naphthalenesulfonic acid was positively correlated with aboveground dry weight per unit area (ADWA, R = 0.54, p < 0.05) and underground dry weight per unit area (UDWA, R = 0.43, p < 0.05), while diethylene glycol was negatively correlated with ADWA (R = -0.34, p < p < 0.05) and UDWA (R = -0.39, p < 0.05), and 2-deoxy-d-glucose was also negatively correlated with ADWA (R = -0.73, p < 0.05) and UDWA (R = -0.58, p < 0.05) (Fig. 8A). 2-deoxy-d-glucose to examine the effects of these three metabolites on the growth of P. notoginseng, a pot experiment was conducted for 6 weeks. After six successive weeks of P. notoginseng treatment (Fig. 8B), the metabolites significantly influenced P. notoginseng plant biomass compared to that in the CK treatment. As shown in the Fig. 8B, there was no significant difference in the biomass of plants treated with 2-naphthalenesulfonic acid or diethylene glycol compared to that of the control, while 2-deoxy-d-glucose reduced the biomass accumulation in the aerial parts of P. notoginseng. A study of the underground biomass of P. notoginseng revealed that 2-naphthalenesulfonic acid promoted underground biomass compared to that of the control; 2-deoxy-d-glucose did not significantly affect the biomass compared to that of the control, while diethylene glycol reduced the biomass accumulation of P. notoginseng (Fig. 8B). These results indicate that the underground plant growth-promoting effects could be attributed to 2-naphthalenesulfonic acid, while 2-deoxy-d-glucose reduced the aboveground biomass. The difference in the biomass of P. notoginseng among the interaction systems may be related to the differences in the levels of these metabolites in the soil. Specifically, the content of 2-deoxy-d-glucose in the CK pots was significantly lower than that in the intercropping pots, and the content of diethylene glycol in the CK pots was significantly lower than that in the intercropping tree species AN and PW; however, the content of 2-naphthalenesulfonic acid in the CK pots was significantly greater than that in the intercropping pots. These findings are consistent with the findings of the seven interaction experiments.
Screening and validation of grown-related metabolites. A Heatmap of the correlation between differentially abundant metabolites and the growth indices. B Effects of soil metabolites on P. notoginseng of aboveground and underground dry weight (Aboveground dry weight of diethylene glycol treatment: F = 0.260, Degrees of Freedom = 3, p > 0.05; Aboveground dry weight of 2-deoxy-d-glucose treatment: F = 12.065, Degrees of Freedom = 3, p < 0.01; Aboveground dry weight of 2-naphthalenesulfonic acid treatment: F = 0.025, Degrees of Freedom = 3, p > 0.05; Underground dry weight of diethylene glycol treatment: F = 3.755, Degrees of Freedom = 3, p < 0.05; Underground dry weight of 2-deoxy-d-glucose treatment: F = 11.980, Degrees of Freedom = 3, p < 0.01; Underground dry weight of 2-naphthalenesulfonic acid treatment: F = 20.321, Degrees of Freedom = 3, p < 0.01). Each cycle represents a biologically independent sample. Horizontal bars within boxes represent the median. The tops and bottoms of boxes represent 75th and 25th quartiles, respectively. Different lowercase letters indicated significant differences among respective groups based on two-sided tests for multiple comparisons by Turkey HSD corrections (ANOVA, adjusted p < 0.05, n = 6 biologically independent samples). Legend: ADW: Aboveground dry weight per plant; UDW: Underground dry weight per plant; ADWA: Aboveground dry weight per unit area; UDWA: Underground dry weight per unit area
Discussion
Intercropping P. notoginseng with trees can contribute to its healthy growth.
In agroforestry systems, intercropping via interactions between different plants can mitigate disease and promote crop growth (Moore et al. 2022; Gong et al. 2019). In this study, interspecific interactions between six tree species and P. notoginseng significantly reduced the incidence of root rot and increased the survival rate of P. notoginseng (Fig. 2). Among these species, the coniferous tree species PO and the broad-leaf tree species ER demonstrated the most effective results. This finding suggested that the interaction between tree species and the root system of P. notoginseng may play a crucial role in reducing the incidence of root rot in forest environments. Similar benefits have been observed in other intercropping systems, e.g., intercropping ginseng in a forest reduced the occurrence of ginseng damping-off disease (Liu et al. 2023). Furthermore, in addition to disease inhibition, the interaction of roots between P. notoginseng and other tree species, especially PO and CL, which are coniferous forest species, also enhanced the germination rate and root biomass of P. notoginseng. Therefore, coniferous forest species may serve as better companions for P. notoginseng than broad-leaved forest species.
Intercropping in agroforestry systems has been proven to enhance crop growth and increase yields, as supported by several studies, such as an increase in the yield of Ginkgo biloba intercropped with Triticum aestivum (Cao et al. 2009) and Malus spectabilis trees intercropped with Glycine max (Wang et al. 2022; Zhou et al. 2023a, 2023b). In the present study, the yield of P. notoginseng significantly increased with the intercropping of PO and CL (Table 2). Similar results have also been reported for forest and mushroom intercropping systems; for example, Lentinula edodes cultivated under Populus L. and Alnus japonica exhibited a greater yield compared with cultivated land with ground cultivation (Tian et al. 2019). Additionally, Huang et al. (2019) and Gao et al. (2020) reported that Bletilla striata and Polygonatum sibiricum had higher yields in a forest-medicine composite system than in a field. Therefore, interactions between species in forest ecosystems significantly contribute to the overall health and well-being of plants.
Improving the quality of P. notoginseng in agroforestry systems through interspecific interactions by intercropping trees
Relevant research has provided compelling evidence for the potential advantages of species intercropping in augmenting crop quality (Zhou et al., 2023a; Wei et al. 2019; Millan et al. 2022; Xu et al. 2022). In our study, we found that PO, CL and SW significantly improved the nutrient concentration of P. notoginseng (Fig. 3). Moreover, the content of the six saponins significantly increased with PO treatment. Similarly, the SW and PW treatments significantly enhanced the content of five saponins, especially saponin R1 (Fig. 4). Therefore, by combining biomass data, we found that PO and SW promoted the growth of P. notoginseng while also increasing its nitrogen, phosphorus, and saponin concentrations. This dual promotion led to a much greater increase in nutrient absorption and saponin accumulation per unit area of P. notoginseng (Parent et al. 2012). Ji et al. (2023) and Wang et al. (2023a, b) reported that nutrient uptake and the notoginsenoside content significantly improved in the cultivation of P. notoginseng in pine forests. Similar studies have also shown that the contents of 20 monomeric saponins, such as Rg1 and Re, significantly increased in P. ginseng cultivated in a Pinus sylvestris var. mongolica forest (Kim et al. 2017; Dai et al. 2020). Some research has suggested that a slight decrease in soil pH promotes the adsorption, dissolution, and release of fixed inorganic phosphorus in the soil and transforms it into available and soluble inorganic phosphorus available to plants (Tian et al. 2020; Long et al. 2016). pH was also the key environmental factor for the synthesis of Rb1 saponins (Moameri et al. 2024; Wang et al. 2023a, b). Our investigation revealed that cultivating P. notoginseng in the understorey led to a decrease in soil pH (Fig. 5), which may result in greater growth and improved quality of P. notoginseng within the forest. Based on the above results, coniferous forest PO and broad-leaved forest SW are also good neighbours of P. notoginseng and can be beneficial to the growth and quality of P. notoginseng.
Regulation of soil metabolism changes by understorey planting promotes the health of P. notoginseng
Soil metabolism regulated by rhizosphere interactions between trees and crops has various functions in interspecies root interactions, except for changing the soil pH (Chen 2022; Yuan et al. 2021; Machiani et al. 2018). In our research, a noteworthy alteration in EC was observed (Fig. 5), this finding suggested that agroforestry with tree species and P. notoginseng has a substantial impact on the diversity and quantity of soil metabolites. Faced with changing environmental conditions, plant metabolism is usually disturbed, and metabolites must be adjusted to maintain basic metabolism, which helps plant hormones respond to stress conditions first (Kosmacz et al. 2020). All initial reactions involve synthesizing and degrading amino acids to produce primary metabolites, such as carbohydrates, proteins, and lipids (Singh et al. 2020). Primary metabolism provides the necessary energy and precursor substances for the biosynthesis of secondary metabolites in the final step (Liu et al. 2017, 2021). In the present study, we utilized HPLC–MS to analyse soil metabolism in a P. notoginseng-tree species interaction experiment and identified 43 differentially abundant metabolites between intercropped P. notoginseng and monocropped P. notoginseng (Fig. 6). The differentially abundant metabolites included lipids, lipid-like molecules, organic acids, derivatives, benzenoids and organoheterocyclic compounds (Fig. S4). In this study, diethylene glycol and 2-deoxy-d-glucose had significant negative relationships with the biomass of P. notoginseng, yet 2-naphthalenesulfonic acid had a positive relationship with the biomass (Fig. 8A). Studies have shown that the use of 2-deoxyglucose as a glycolytic inhibitor renders fungi unusable (Zhou et al. 2011). Ji et al. (2024) found that the antibacterial agent diglycolate, which is the active ingredient, could inhibit the growth of wheat, but there are few reports on the use of 2-deoxyglucose and diethylene glycol for promoting production. Our pot experiment confirmed that increasing 2-naphthalenesulfonic acid concentrations or decreasing diethylene glycol and 2-deoxy-d-glucose concentrations is beneficial for the growth of P. notoginseng (Fig. 8B). Therefore, soil metabolic changes mediated by tree species roots may be an important mechanism for the growth advantages observed in P. notoginseng understorey plantings (Xu et al. 2020).
Studies have shown that the metabolites in the rhizosphere soil of Panax quinquefolius, such as lipids (Wu and Lin 2020), organic acids, sugars, etc., can provide carbon (Li et al. 2022a, b) and nitrogen (Zhou et al. 2023b, a) for soil microorganisms and promote root adaptation to soil environmental changes, affecting the healthy growth of plants (Guo et al. 2023). When pathogens invade plant roots, plant growth can be affected by inhibiting, promoting, and causing the chemotaxis of rhizosphere metabolites (Zhang et al. 2019a, b; Lei et al. 2018). Through correlation analysis with the growth indices of P. notoginseng and through plate confrontation and pot experiments, we determined that indole-3-carbaldehyde was beneficial for reducing the occurrence of root rot in P. notoginseng, while 2-naphthalenesulfonic acid was positively correlated with disease (Fig. 7). The synthesis of indole-3-carbaldehyde compounds derived from indoles has received significant attention in the medical field (Metwally et al. 2010). This is due to the broad-spectrum biological activities of indole-3-carbaldehyde, such as antibacterial activity (Xu et al. 2011) and anti-inflammatory activity (Rani et al. 2004). We also found that 2-naphthalenesulfonic acid is an important intermediate for the production of dyes, pesticides and drugs (Lin et al. 1988). However, limited research has been conducted on the inhibition of plant pathogens by indole-3-acyl compounds or 2-naphthalenesulfonic acid. Only substituted indole-3-carboxaldehyde compounds, which have attracted worldwide attention due to their unique chemical structure and extensive physiological and pharmacological activities, have been demonstrated to effectively inhibit Exserohilum turcicum and Helminthosporium maydis (Che et al. 2015). However, there is little direct research on the effects of this fungus on resistance to pathogenic bacteria or plant growth. Our pot interaction experiment confirmed these findings by revealing a greater relative abundance of indole-3-carboxaldehyde in the soil metabolism of PO and CL and the opposite trend for 2-naphthalenesulfonic acid. Therefore, these findings may help to elucidate the disease resistance mechanism of the interaction system of these three species with P. notoginseng.
Moreover, numerous studies had documented changes in the composition of rhizosphere metabolites in response to various neighbouring plants (Wen et al. 2023b, 2023c). This interaction contributed to the development of disease-suppressive soils by recruiting beneficial microbial populations such as Bacillus subtilis and Pseudomonas strains (Wen et al. 2021; Li and Jiang 2021; Haichar et al. 2014). Although the exact mechanisms remained elusive, these findings highlight the complex relationship between root metabolites and microorganisms. In the future, we aimed to conduct more detailed research to further elucidate the relationship between root metabolites and microorganisms, and their role in plant disease resistance.
Conclusion
The occurrence of root rot in P. notoginseng plants was significantly reduced when intercropped with PO and SW, resulting in enhanced growth, nutrient, and saponin concentrations. Additionally, indole-3-carboxaldehyde, a compound present in intercropping soil, was instrumental in mitigating the root rot of P. notoginseng. Simultaneously, compound such as 2-naphthalenesulfonic acid, also found in intercropping soil, was increased the development and quality of growth in P. notoginseng. These findings suggest that soil metabolic alterations may underlie the observed growth and disease resistance benefits in the understorey cultivation of P. notoginseng. Consequently, Platycladus orientalis (coniferous forests) and Schima wallichii Choisy (broad-leaved forests) emerged as potentially beneficial companions for P. notoginseng.
Data availability
All Raw sequencing data have been submitted to the NCBI GenBank accession number(s) for your nucleotide sequence(s) under the accession number PP493919 and PP514759.
References
Abdalmoula MM, Makineci E, Özturna AG, Pehlivan S, Şahin A, Tolunay D (2019) Soil organic carbon accumulation and several physicochemical soil properties under stone pine and maritime pine plantations in coastal dune, Durusu-Istanbul. Environ Monit Assess 191:312–326. https://doi.org/10.1007/s10661-019-7472-6
Bélanger G, Castonguay Y, Lajeunesse J (2014) Benefits of mixing timothy with alfalfa for forage yield, nutritive value, and weed suppression in northern environments. Can J Plant Sci 94:51–60. https://doi.org/10.4141/CJPS2013-228
Boudreau Mark A (2013) Diseases in intercropping systems. Annu Rev Phytopathol 51:499–519. https://doi.org/10.1146/annurev-phyto-082712-102246
Cao FL, Kimmins JP, Jolliffe PA, Wang JR (2009) Relative competitive abilities and productivity in Ginkgo and broad bean and wheat mixtures in southern China. Agrofor Syst 79:369–380. https://doi.org/10.1007/s10457-009-9268-0
Che ZP, Liu SM, Wei SL, Chen GQ (2015) Synthesis and Antifungal Activities of 3-Formylindoles against Exserohilum turcicum and Helminthosporium maydis in vitro. Agrochemicals 54:177–179. https://doi.org/10.16820/j.cnki.1006-0413.2015.03.005
Chen MX (2022) Effects of maize-soybean intercropping patterns on improving crop quality and ecological benefits. Seed Sci Technol 40:29–31. https://doi.org/10.19904/j.cnki.cn14-1160/s.2022.22.009
Chen JW, Kuang SB, Long GQ, Meng ZG, Li LG, Chen ZJ, Zhang GH, Yang SC (2014) Steady-state and dynamic photosynthetic performance and nitrogen partitioning in the shade-demanding plant Panax notoginseng under different levels of growth irradiance. Acta Physiol Plant 36:2409–2420. https://doi.org/10.1007/s11738-014-1614-9
Chen JY, Wang WT, Kong JQ, Yue YD, Dong YY, Zhang JC, Liu L (2022) Application of UHPLC-Q-TOF MS based untargeted metabolomics reveals variation and correlation amongst different tissues of Eucommia ulmoides Oliver. Microchem J 172. https://doi.org/10.1016/j.microc.2021.106919
Chen YF (2023) Rhizosphere microbiome analysis and selection and construction of growthpromoting flora of maize and soybean under single and intercropping. Northwest A&F University. https://doi.org/10.27409/d.cnki.gxbnu.2023.000329
Cui X, Huang L, Guo L, Liu D (2014) Chinese Sangi industry status and development countermeasures. China J Chin Materia Med 39(4):553–557. https://doi.org/10.4268/cjcmm20140401
Dai YL, Qiao MD, Yu P, Zheng F, Yue H, Liu SY (2020) Comparing eight types of ginsenosides in ginseng of different plant ages and regions using RRLC-Q-TOF MS/MS. J Ginseng Res 44:205–214. https://doi.org/10.1016/j.jgr.2017.11.001
Deng PF, Yin RY, Wang HL, Chen LR, Cao XQ, Xu XN (2023) Comparative analyses of functional traits based on metabolome and economic traits variation of Bletilla striata: Contribution of intercropping. Frontiers in Plant Science 14:114–7076. https://doi.org/10.3389/fpls.2023.1147076
Dong XM, Gao XL, Liu W, Li GX, Li M, Zhang AN (2021) Research progress of autotoxicity in continuous cropping obstacle of peach. Heilongjiang Agric Sci 1(2):123–127. https://doi.org/10.11942/j.issn1002-2767.2021.02.0123
Du Y, Song JY, Zhang YL, Chai XT, Zhang ZH, WaqarI SLAM , Zeng FJ (2024) Effects of Mowing on Between Leaf Internal Stability and Leaf and Soil Stoichiometric Characteristics of Legumes and Non-legumes. Acta Agrestia Sinica 1–10. https://link.cnki.net/kcms/detail/11.3362.S.20240315.1538.006
Finn JA, Kirwan L, Connolly J, Sebastià MT, Helgadottir A, Baadshaug OH, Bélanger G, Black A, Brophy C, Collins RP, Čop J, Dalmannsdóttir S, Delgado I, Elgersma A, Fothergill M, Lindberg BEF, Ghesquiere A, Golinska B, Golinski P, Grieu P, Gustavsson AM, Höglind M, Elie OH, Jørgensen M, Kadziuliene Z, Kurki P, Llurba R, Lunnan T, Porqueddu C, Suter M, Thumm U, Luscher A (2013) Ecosystem function enhanced by combining four functional types of plant species in intensively managed grassland mixtures: a 3-year continental-scale field experiment. J Appl Ecol 50(2):365–375. https://doi.org/10.1111/1365-2664.12041
Gaba S, Lescourret F, Boudsocq S, Enjalbert J, Hinsinger P, Journet EP, Navas ML, Wery J, Louarn G, Malézieux E, Pelzer E, Prudent M, Lafontaine HO (2015) Multiple cropping systems as drivers for providing multiple ecosystem services: from concepts to design. Agron Sustain Dev 35:607–623. https://doi.org/10.1007/s13593-014-0272-z
Gao WX, Shi TY, Zhou QH, Duan FW, Chen XX (2020) Experiment on high-yield cultivation of understory Bletilla striata and Polygonatum kingianum in chuxiong. For Invent Plan 45(2):119–123. https://doi.org/10.3969/j.issn.1671-3168.2020.02.021
Ge S, Pang SJ, Peng WT, Fang W, Wang Y, Liu R, Gan QY, Qi WT (2024) Fructose aggravating colon barrier dysfunction by decreasing gut bacteria metabolites indole-3-carboxaldehyde and inhibiting activation of aryl hydrocarbon receptor in vivo and in vitro. Food Science and Human Wellness 1–33. https://link.cnki.net/urlid/10.1750.TS.20240228.1638.004
Gong XW, Liu CJ, Li J, Luo Y, Yang QH, Zhang WL, Yang P, Feng BL (2019) Responses of rhizosphere soil properties, enzyme activities and microbial diversity to intercropping patterns on the Loess Plateau of China. Soil Till Res 195:104355. https://doi.org/10.1016/j.still.2019.104355
Guay MOM, Paquette A, Dupras J, Rivest D (2018) The new Green Revolution: Sustainable intensification of agriculture by intercropping. Sci Total Environ 615:767–772. https://doi.org/10.1016/j.scitotenv.2017.10.024
Guo RQ, Guan RW, Hu CM, Zhao QC, Lv YJ, Lin HB, Li X (2023) Analysis of soil metabolite accumulation in rhizosphere based on LC-MS for American ginseng. Lishizhen Med Mater Med Res 34(08):1976–1980. https://doi.org/10.3969/j.issn.1008.0805.2023.08.49
Haichar FZ, Santaella C, Heulin T, Achouak W (2014) Root exudates mediated interactions belowground. Soil Biol Biochem 77:69–80. https://doi.org/10.1016/j.soilbio.2014.06.017
Hei JY, Wang S, He XH (2023) Effects of exogenous organic acids on the growth, edaphic factors, soil extracellular enzymes, and microbiomes predict continuous cropping obstacles of Panax notoginseng from the forest understorey. Plant and Soil. https://doi.org/10.1007/s11104-023-06044-0
Hu HY, Li H, Hao MM, Ren YN, Zhang MK, Liu RY, Zhang Y, Li G, Chen JS, Ning TY, Kuzyakov Y (2021) Nitrogen fixation and crop productivity enhancements co-driven by intercrop root exudates and key rhizosphere bacteria. J Appl Ecol 58:2243–2255. https://doi.org/10.1111/1365-2664.13964
Huang XJ, Zhang WB, Lai GW, Zhang FG, Jiang ZH, Qiu WQ (2019) Effects of different shading degrees on the growth of Bletilla striata and Rhizoma huangjing under compound management. Modern Agric Res 22:49–51. https://doi.org/10.19704/j.cnki.xdnyyj.2019.04.022
Ji C, Lu YX, Li J, Hua MZ, Xie YX, Ma Y, Shi R, Zhao LJ, Yang M, He XH, Zheng WJ, Lu XN (2023) Determination of Dencichine in Panax notoginseng in the Forest and Field Using High Performance Liquid Chromatography. ACS Omega 8:27450–27457. https://doi.org/10.1021/acsomega.3c02962
Ji DX, Tang Y, Tan H, Jin Z (2024) Preparation and application of a diglycolate antibacterial agent. Chinese Patent: CN 115316389 A, November 11, 2022
Jia WJ, Wang S, He XH, Zhao XY (2022) Different factors drive the assembly of pine and Panax notoginseng-associated microbiomes in Panax notoginseng-pine agroforestry systems. Front Microbiol 13:1018989. https://doi.org/10.3389/fmicb.2022.1018989
Kim JH, Yi YS, Kim MY, Cho JY (2017) Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J Ginseng Res 41:435–443. https://doi.org/10.1016/j.jgr.2016.08.004
Kosmacz M, Sokołowska EM, Bouzaa S, Skirycz A (2020) Towards a functional understanding of the plant metabolome. Curr Opin Plant Biol 55:47–51. https://doi.org/10.1016/j.pbi.2020.02.005
Lei FJ, Zhang AH, Fu JF, Wang Z, Zhang LX (2018) Effect of total ginsenoside on 2 kinds of vegetable pathogens. Modern Chin Med 20(1):70–73. https://doi.org/10.13313/j.issn.16734890.20170928002
Li L, Jiang JL (2021) Analysis of specific metabolites in rhizosphere soil of Panax quinquefolius L. with root rot diseases based on metabolomics. Mol Plant Breed 19(18):6012–6019. https://doi.org/10.13271/j.mpb.019.006012
Li CJ, Hoffland E, Kuyper TW, Yu Y, Zhang CC, Li HG, Zhang FS, Werf W (2020a) Syndromes of production in intercropping impact yield gains. Nat Plants 6:653–660. https://doi.org/10.1038/s41477-020-0680-9
Li LL, Zhao HH, Kong CH (2020b) (-)-Loliolide, the most ubiquitous lactone, is involved in barnyardgrass-induced rice allelopathy. J Exp Bot 71:1540–1550. https://doi.org/10.1093/jxb/erz497
Li L, Jiang JL, Dong YX, Shu Y (2022a) Allelochemical substances screening and their allelopathic effects analysis in rhizosphere soil of continuous cropping american ginseng. Acta Agric Boreali-Occidentalis Sinica 31:1046–1057. https://doi.org/10.7606/j.issn.1004-1389.2022.08.012
Li M, Pommier T, Yin Y, Wang JN, Gu SH, Jousset A, Keuskamp J, Wang HG, Wei Z, Xu YC, Shen QR, Kowalchuk GA (2022b) Indirect reduction of Ralstonia solanacearum via pathogen helper inhibition. ISME J 16:868–875. https://doi.org/10.1038/s41396-021-01126-2
Li TY, Ye C, Zhang YJ, Zhang JX, Yang M, He XH, Mei XY, Liu YX, Zhu YY, Huang HC, Zhu SS (2023) 2,3-Butanediol from the leachates of pine needles induces the resistance of Panax notoginseng to the leaf pathogen Alternaria panax. Plant Diversity 45:104–116. https://doi.org/10.1016/j.pld.2022.02.003
Lin CS, Zhang XS, He TJ, He L, Yin F (1988) On the Determination of 1-Naphthalenesulphonic Acid by Reversed-Phase High Performance Liquid Chromatography. Chromatography 6(05):285–288
Liu J, Liu Y, Wang Y, Abozeid A, Zu YG, Tang ZH (2017) The integration of GC–MS and LC-MS to assay the metabolomics profiling in Panax ginseng and Panax quinquefolius reveals a tissue- and species-specific connectivity of primary metabolites and ginsenosides accumulation. J Pharm Biomed Anal 135:176–185. https://doi.org/10.1016/j.jpba.2016.12.026
Liu HJ, Gu HR, Ye C, Guo CW, Zhu YF, Huang HC, Liu YX, He XH, Yang M, Zhu SS (2021) Planting density affects Panax notoginseng growth and ginsenoside accumulation by balancing primary and secondary metabolism. Front Plant Sci 12:628294. https://doi.org/10.3389/fpls.2021.628294
Liu ZY, Zhang QY, Yang P, Lu BH, Yang H, Xu YH (2023) Isolation and identification of endophytic biocontrol bacteria from understory Panax ginseng and study on colonization ability. Microbiology China 50:2519–2531. https://doi.org/10.13344/j.microbiol.china.220894
Long M, Wu HH, Smith MD, Pierre KJL, Lv XT, Zhang HY, Han XG, Yu Q (2016) Nitrogen deposition promotes phosphorus uptake of plants in a semi-arid temperate grassland. Plant Soil 408:475–484. https://doi.org/10.1007/s11104-016-3022-y
Lu R (2000) Analysis methods of soil agricultural chemistry. Chinese Agricultural Science and Technology Press, Beijing
Luo LF, Wang LT, Deng LM, Mei XY, Liu YX, Huang HC, Du F, Zhu SS, Yang M (2021) Enrichment of Burkholderia in the rhizosphere by autotoxic ginsenosides to alleviate negative plant-soil feedback. Microbiology Spectrum 9:e0140021. https://doi.org/10.1128/Spectrum.01400-21
Lv HF, Cao HS, Nawaz MA, Sohail H, Huang Y, Cheng F, Kong QS, Bie ZL (2018) Wheat intercropping enhances the resistance of watermelon to fusarium wilt. Front Plant Sci 9:696. https://doi.org/10.3389/fpls.2018.00696
Machiani MA, Javanmard A, Morshedloo MR, Maggi F (2018) Evaluation of yield, essential oil content and compositions of peppermint (Mentha piperita L.) intercropped with faba bean (Vicia faba L.). J Clean Prod 171:529–537. https://doi.org/10.1016/j.jclepro.2017.10.062
Malézieux E, Crozat Y, Dupraz C, Laurans M, Makowski D, Lafontaine HO, Rapidel B, Tourdonnet S, Morison MV (2009) Mixing plant species in cropping systems: concepts, tools and models. A review. Agron Sustain Dev 29:43–62. https://doi.org/10.1051/agro:2007057
Metwally MA, Shaaban S, Wahab BFA, Hiti GAE (2010) ChemInform Abstract: 3-Acetylindoles: Synthesis, Reactions and Biological Activities. Curr Org Chem 41(20):1475–1496. https://doi.org/10.1002/chin.201020214
Millan AFS, Gamir J, Larraya L, Farran I, Veramendi J (2022) Towards understanding of fungal biocontrol mechanisms of different yeasts antagonistic to Botrytis cinerea through exometabolomic analysis. Biol Contr 55:47–51. https://doi.org/10.1016/j.biocontrol.2022.105033
Moameri M, Ghorbani A, Dadjou F, Biswas A, Varasteh F (2024) Soil and plant attributes under grazing exclusion in semi-steppic semi-arid rangelands, Iran. Arid Land Res Manag 1–19. https://doi.org/10.1080/15324982.2023.2299017
Moore VM, Schlautman B, Fei SZ, Roberts LM, Wolfe M, Ryan MR, Wells S, Lorenz AJ (2022) Plant breeding for intercropping in temperate field crop systems: A Review. Front Plant Sci 13. https://doi.org/10.3389/fpls.2022.843065
Parent SE, Parent LE, Rozanne DE, Hernandes A, Natale W (2012) Nutrient balance as paradigm ofsoil and plant chemometricsNutrient Balance as Paradigm of Soil and Plant Chemometrics. Soil Fertility Chapter 4. https://doi.org/10.5772/53343
Peng Z, Guo XZ, Xu Y, Liu DH, Wang HY, Guo LP, Zhang Y (2020) Advances in interaction between medicinal plants and rhizosphere microorganisms. China J Chin Materia Med 45:2023–2030. https://doi.org/10.19540/j.cnki.cjcmm.20200302.116
Rani P, Srivastava VK, Kumar A (2004) Synthesis and antiinflammatory activity of heterocyclic indole derivatives. Eur J Med Chem 39:449–452. https://doi.org/10.1016/j.ejmech.2003.11.002
Singh BK, Trivedi P, Egidi E, Macdonald CA, Baquerizo MD (2020) Crop microbiome and sustainable agriculture. Nat Rev Microbiol 18:601–602. https://doi.org/10.1038/s41579-020-00446-y
Teng Y, Ren Y, Sayed M, Hu X, Lei C, Kumar A, Hutchins E, Mu J, Deng Z, Luo C, Sundaram K, Sriwastva MK, Zhang L, Hsieh M, Reiman R, Haribabu B, Yan J, Jala VR, Miller DM, Jensen KVK, Merchant ML, McClain CJ, Park JW, Egilmez NK, Zhang HG (2018) Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe 24:637-652.e638. https://doi.org/10.1016/j.chom.2018.10.001
Tian Y, Song S, Niu Y, Yang L, Liu Y, Huang Z, Wang S (2019) Comparative analysis of stand and field planting patterns of Lentinula edodes in woodland. Jiangsu Agric Sci 47:168–171. https://doi.org/10.15889/j.issn.10021302.2019.18.036
Tian MY, Yu CJ, Wang JK, Ding F, Chen ZH, Jiang N, Jiang H, Chen LJ (2020) Effect of nitrogen additions on soil pH, phosphorus contents and phosphatase activities in grassland. Chin J Appl Ecol 31:2985–2992. https://doi.org/10.13287/j.1001-9332.202009.034
Wan Y, Zhao X, Zhou D, Chen S, Li Z (2018) Compound Planting of Bletilla striata under Forest in the Three Gorges Dam Area. Agricultural Biotechnology 7:203–206
Wang Z (2023) Discussion on the planting technology of Polygonatum cyrtonema forest. Southern Agric Mach 54:70–73. https://doi.org/10.3969/j.issn.1672-3872.2023.20.019
Wang F, Sun WS, Zhang XT, Yi LF, Zhu LX, Ge CM, Sun MT (2019) Effects of intercropping Allium fistulosum on root exudates of Platycodon grandiflorum. Shandong Agric Sci 51:68–73. https://doi.org/10.14083/j.issn.1001-4942.2019.11.015
Wang XY, Yang T, Shen L, Zhang WL, Wan SM, Zhang W, Li LH (2021a) Formation of factors influencing cotton yield in jujube–cotton intercropping systems in Xinjiang, China. Agrofor Syst 95:177–189. https://doi.org/10.1007/s10457-020-00571-w
Wang Y, He S, Xiong BJ, Wang HL, Jia LY, He XH, Shi R (2021b) Studied on microbial diversity of rhizosphere soil from different cultivated Panax ginseng. China Tradit Herbal Drugs 52:5303–5310. https://doi.org/10.7501/j.issn.0253-2670.2021.17.023
Wang Z, Chen Z, Tang Y, Zhang M, Huang M (2023a) Regulation of transcriptome networks that mediate ginsenoside biosynthesis by essential ecological factors. PLoS ONE 18:e0290163. https://doi.org/10.1371/journal.pone.0290163
Wang Z, Wang W, Yang K, Ye C, Wu W, Wang C, Mao G, Huang H, Mei X, Yang M, Zhu S, Zhu Y, He X, Liu Y (2023b) Adult-plant resistance of Panax notoginseng to nematodes and interspecific facilitation with pine trees. J Pest Sci 96:1271–1286. https://doi.org/10.1007/s10340-023-01601-z
Wang XY, Shen L, Liu TT, Wei WW, Zhang S, Li LH, Zhang W (2022) Microclimate, yield, and income of a jujube–cotton agroforestry system in Xinjiang. China. Ind Crops Prod 182. https://doi.org/10.1016/j.indcrop.2022.114941
Wei JH, Wang RH, Liu KL, Chen GM, Fu WN, Liu JT (2019) Benefit analysis of Aleurites montana forest-medicinal intercropping in southern Hunan. Non-Wood For Res 37:141–147. https://doi.org/10.14067/j.cnki.1003-8981.2019.02.020
Wen T, Zhao ML, Yuan J, Kowalchuk GA, Shen QR (2021) Root exudates mediate plant defense against foliar pathogens by recruiting beneficial microbes. Soil Ecol Lett 3:42–51. https://doi.org/10.1007/s42832-020-0057-z
Wen T, Xie PH, Penton CR, Hale L, Thomashow LS, Yang SD, Ding ZX, Su YQ, Yuan J, Shen QR (2022) Specific metabolites drive the deterministic assembly of diseased rhizosphere microbiome through weakening microbial degradation of autotoxin. Microbiome 10:177. https://doi.org/10.1186/s40168-022-01375-z
Wen T, Ding ZX, Thomashow LS, Hale L, Yang SD, Xie PH, Liu XY, Wang HQ, Shen QR, Yuan J (2023a) Deciphering the mechanism of fungal pathogen-induced disease-suppressive soil. New Phytol 238:2634–2650. https://doi.org/10.1111/nph.18886
Wen T, Niu GQ, Chen T, Shen QR, Yuan J, Liu YX (2023b) The best practice for microbiome analysis using R. Protein Cell 14:713–725. https://doi.org/10.1093/procel/pwad024
Wen T, Xie PH, Liu HW, Liu T, Zhao ML, Yang SD, Niu GQ, Hale L, Singh BK, Kowalchuk GA, Shen QR, Yuan J (2023c) Tapping the rhizosphere metabolites for the prebiotic control of soil-borne bacterial wilt disease. Nat Commun 14. https://doi.org/10.1038/s41467-023-40184-2
Weyers SL, Gesch RW, Forcella F, Eberle CA, Thom MD, Matthees HL, Ott M, Feyereisen GW, Strock JS (2021) Surface runoff and nutrient dynamics in cover crop-soybean systems in the Upper Midwest. J Environ Qual 50:158–171. https://doi.org/10.1002/jeq2.20135
Wu HM, Lin WX (2020) A commentary and development perspective on the consecutive monoculture problems of medicinal plants. Chin J Eco-Agric 28:775–793. https://doi.org/10.13930/j.cnki.cjea.190760
Xiao F (2023) Effects of exogenous IAA application on Pb enrichment in the intercropping of Arabis alpina L. Var parviflora Franch and Zea mays L. Yunnan Agricultural University. https://doi.org/10.27458/d.cnki.gynyu.2023.000641
Xiong B, Shi R, He S, Li J, Wang Y, Jia L, He X (2022) Effects of secondary metabolites of Panax notoginseng under forest in different cultivation modes. J Shenyang Pharm Univ 1–12. https://doi.org/10.14066/j.cnki.cn21-1349/r.2021.0596
Xu H, Yang W, Wang Q (2011) Antifungal agents. Part 3: synthesis and antifungal activities of 3-acylindole analogs against phytopathogenic fungi In Vitro. Chem Biol Drug Des 78:864–868. https://doi.org/10.1111/j.1747-0285.2011.01212.x
Xu YG, Liu HJ, Zhang KM, Zhu SS, Yang M (2020) Allelopathic plants : 28. Genus Panax l. Allelopathy J 51:21–40. https://doi.org/10.26651/allelo.j/2020-51-1-1288
Xu F, Lv Z, Yang L, Han M, Yang L (2022) Effects of ecological planting of tree-herb intercropping on yield and quality of Saposhnikovia divaricata (Turcz.) Schischk. J Jilin Agric Univ. https://cnki.net/kcms/detail/22.1100.S.20220505.1338.002
Yang M, Zhang Y, Qi L, Mei XY, Liao JJ, Ding XP, Deng WP, Fan L, He XH, Vivanco JM, Li CY, Zhu YY, Zhu SS (2014) Plant-plant-microbe mechanisms involved in soil-borne disease suppression on a maize and pepper intercropping system. PLoS ONE 9:e115052. https://doi.org/10.1371/journal.pone.0115052
Yang M, Chuan YC, Guo CW, Liao JJ, Xu YG, Mei XY, Liu YX, Huang HC, He XH, Zhu SS (2018) Panax notoginseng root cell death caused by the autotoxic ginsenoside Rg1 is due to over-accumulation of ROS, as revealed by transcriptomic and cellular approaches. Front Plant Sci 9:264. https://doi.org/10.3389/fpls.2018.00264
Yang K, Wang HL, Luo LF, Zhu SS, Huang HP, Wei ZX, Zhu YY, Guo LW, He XH (2022) Effects of different soil moisture on the growth, quality, and root rot disease of organic Panax notoginseng cultivated under pine forests. J Environ Manage 329:117069. https://doi.org/10.1016/j.jenvman.2022.117069
Yang Y, Chen MJ, Zhang W, Zhu HY, Li H, Niu XJ, Zhou ZS, Hou XY, Zhu JL (2023) Metabolome combined with transcriptome profiling reveals the dynamic changes in flavonoids in red and green leaves of Populus × euramericana ‘Zhonghuahongye’. Front Plant Sci 14:1274700. https://doi.org/10.3389/fpls.2023.1274700
Ye C, Fang HY, Liu HJ, Yang M, Zhu SS (2019) Current status of soil sickness research on Panax notoginseng in Yunnan, China. Allelopath J 47:1–14. https://doi.org/10.26651/allele.j/2019-47-1-1216
Ye C, Liu YX, Zhang JX, Li TY, Zhang YJ, Guo CW, Yang M, He XH, Zhu YY, Huang HC, Zhu SS (2021) α-Terpineol fumigation alleviates negative plant-soil feedbacks of Panax notoginseng via suppressing Ascomycota and enriching antagonistic bacteria. Phytopathol Res 3:1–17. https://doi.org/10.1186/s42483-021-00090-1
Yuan T, Li X, Gong XY, Shi JB, Tao M, Wu FZ (2021) Effects of early spring greenhouse bean / celery intercropping on its yield and quality. Northern Horticult 18:42–47. https://doi.org/10.11937/bfyy.20210120
Zhang H, He YY, Wu JQ, Yang YX, Zheng KY, Yang M, Zhu SS, He XH, Zhu YY, Liu YX (2019a) Inhibitory activity of key antifungal substances in maize root exudates against Phytophthora. sojae. Plant Prot 45:124–130. https://doi.org/10.16688/j.zwbh.2018487
Zhang XX, Zhang AH, Lei FJ, Cai L, Xu ZY, Liu ZQ, Zhang LX (2019b) Chemotactic response of ginseng endophyte to ginseng root exudates. China J Chin Materia Med 44:5358–5362. https://doi.org/10.19540/j.cnki.cjcmm.20191009.105
Zhang R, Yan XQ, Yang ZL, Zhang DD, Yan MX, Wang YP (2023a) Advances in study on intercropping of crops. Spec Wild Econ Anim Plant Res 638–645. https://doi.org/10.16720/j.cnki.tcyj.2023.171
Zhang S, Shen L, Wang XY, Liu TT, Wei WW, Li LH, Wang JP, Cheng ZB, Zhang W (2023b) Research progress on agroforestry systems in xinjiang. Oasis Agric Sci Eng 64–72
Zhou XH, Lu ZX, Lv FX, Bie XM (2011) Bio-control effect of combining Bacillus subtilis fmbj with 2-deoxy-d-glucose on Rhizopus stolonifer. Sci Agric Sin 44(21):4447–4453. https://doi.org/10.3864/j.issn.0578-1752.2011.21.013
Zhou MQ, Sun CL, Dai B, He Y, Zhong J (2023a) Intercropping system modulated soil–microbe interactions that enhanced the growth and quality of flue-cured tobacco by improving rhizospheric soil nutrients, microbial structure, and enzymatic activities. Front Plant Sci 14:1233464. https://doi.org/10.3389/fpls.2023.1233464
Zhou X, Zhang J, Khashi URM, Gao D, Wei Z, Wu F, Dini-Andreote F (2023b) Interspecific plant interaction via root exudates structures the disease suppressiveness of rhizosphere microbiomes. Mol Plant 16:849–864. https://doi.org/10.1016/j.molp.2023.03.009
Zhu L, Yan H, Liu P, Zhang ZY, Zhang S, Guo S, Jiang S, Duan JA (2021) Research progress on effects of rhizosphere microorganisms on quality formation of medicinal plants and their interaction mechanisms. Chin Herbal Med 52:4064–4073. https://doi.org/10.7501/j.issn.0253-2670.2021.13.030
Zhu SS, Huang HC, Liu YX, Li CY, He XH, Zhu YY (2022) Research progress of agricultural biodiversity in preventing and controlling crop diseases. J Plant Propect 49:42–57. https://doi.org/10.13802/j.cnki.zwbhxb
Funding
This work was supported by the National Natural Science Foundation of China (U23A20202, 32260706, 32202400), the Natural Science Foundation of Yunnan Province (202301BD070001-022), the Expert Workstation Project in Yunnan Province (202305AF140327), the Young and Middle-aged Academic and Technical Leaders Reserve Programme in Yunnan Prov-ince (202105AC160069), and Yunnan High-level Personnel Training Program Young and Elite Talents Project (YNWR-QNBJ-2020–285).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Cuiying Wang, Guomin Mao, Yingbin Li, Wenjing Zi, Qingying Wang original researcher, introduction, methodologist, statistical analyst, writing- review and editing; Huichuan Huang, Min Yang, Fei Du, Xinyue Mei, Weiping Deng, Jian Lu methodologist, statistical analyst; Cuiying Wang sampling, methodologist; Shusheng Zhu, Chen Ye, Yixiang Liu, writing- review and editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: Rémi Cardinael.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Fig. 1. Fusarium solani Pathogenicity Detection Procedure. Supplementary Fig. 2. Classification plots of the 630 soil metabolites. Supplementary Fig. 3. Analysis of Relative Content of 43 Soil Metabolites. Supplementary Fig. 4. Classification plots of the 43 differential metabolites.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, C., Mao, G., Li, Y. et al. Tree root-mediated soil metabolome in agroforestry enhancing the growth and quality of Panax notoginseng. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06744-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06744-1