Abstract
Aims
The transcription factors bZIP19 and bZIP23 function as central regulators of the Zn deficiency response, and also as sensors of intracellular Zn concentration through their protein Zn-Sensor Motif (ZSM). While under Zn deficiency the target genes of bZIP19/23 are transcriptionally activated, under Zn sufficiency the binding of Zn2+ ions to the ZSM halts gene expression. Mutations, including deletions, in the ZSM affect the activity of bZIP19/23 and leads to a Zn-insensitive and constitutive activation of target gene expression. Here we investigated the effects of such deregulation of the Zn deficiency response on plant growth and Zn accumulation, and evaluate whether this deregulation influences Cd accumulation.
Methods
We analysed Arabidopsis lines constitutively expressing bZIP19 with the ZSM deleted and measured developmental traits and ionomics in soil-grown plants, comparing control and Cd-spiked soils.
Results
Results indicated that deletion of the ZSM, and the consequent deregulation of the Zn deficiency response, does not cause visible penalties in plant growth, development or reproduction. Compared with the wild-type, bZIP19-ZSM deletion increased Zn accumulation in leaves and seeds, and such an increase was mostly limited to Zn. In seeds, the increased Zn content appears distributed evenly throughout the embryo. Exposure of bZIP19-ZSM deletion to a low-level Cd contamination did not cause enhanced Cd accumulation, which is important given that Cd uptake is a concern in crop Zn biofortification. Finally, we verified that the bZIP19-ZSM deletion represents a gain-of-function dominant mutation.
Conclusion
Together, results support that modulation of F-bZIP transcription factor’s activity may be a promising avenue for Zn biofortification in crops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zinc (Zn) is an essential micronutrient for all living organisms, and it is a key structural or catalytic component for a large number of proteins, with around 10% of the total proteome of eukaryote organisms estimated to comprise Zn-binding proteins (Andreini et al. 2006). Zn deficiency is predicted to affect up to 20% of the world’s population (Wessells and Brown 2012). In humans, Zn deficiency negatively affects development, growth, and immune system function (Prasad 2009). Most of those who are afflicted depend on a plant-based diet consisting of staple-crops grown on Zn-deficient soils (Welch and Graham 2004). Also, the increased popularity of vegetarian diets raises concerns of micronutrient deficiencies including Zn (Assunção et al. 2022).
To improve the Zn content of crops it is necessary to understand the molecular basis of plant Zn homeostasis. By maintaining a Zn concentration within physiological limits, and avoiding deficiency or toxicity, plants rely on a tightly regulated network of homeostasis mechanisms (Clemens 2001; Castro et al. 2018). Such mechanisms include the movement of Zn across membranes by transporters, and interaction of Zn with ligands that modify its mobility and availability. These processes are regulated in response to plant Zn status. In Arabidopsis thaliana (Arabidopsis) the partially redundant BASIC LEUZINE-ZIPPER (bZIP) transcription factors bZIP19 and bZIP23 are the central regulators of the Zn deficiency response. Under Zn deficiency, bZIP19/23 bind to Zinc Deficiency Response Elements (ZDREs) that are present in promotors of their target genes, and activate transcription (Assunção et al. 2010). These target genes consist of a small set of Zn homeostasis genes involved in Zn transport and distribution. They encode Zn transporters from the ZRT/IRT-like protein (ZIP) family that mediate Zn uptake into the cell, and nicotianamine synthase (NAS) enzymes that produce the low-molecular-weight Zn ligand nicotianamine, which is involved in Zn intercellular and long-distance movement (Guerinot 2000; Clemens et al. 2013b).
The transcription factors bZIP19 and bZIP23 not only function as central regulators of the Zn deficiency response, but also as sensors of intracellular Zn concentration through a Zn-Sensor Motif (ZSM) located at the protein N-terminus. Under Zn deficiency, the target genes of bZIP19/23 are transcriptionally activated, but under Zn sufficiency, the binding of Zn2+ ions to the ZSM halts the gene transcriptional activation (Lilay et al. 2021). This is possibly due to a protein conformational change upon Zn binding that affects the dimerization and/or DNA-binding of bZIP19/23 (Assunção et al. 2013). Mutations in the ZSM, which comprises two short regions rich in cysteine (Cys) and histidine (His) amino acids, lead to a Zn-insensitive variant of bZIP19/23 and constitutive expression of its target genes. Plants with such deregulation of the bZIP19/23-regulated Zn deficiency response, grown under Zn sufficient supply, showed increased Zn accumulation compared with the wild-type (Lilay et al. 2021). This suggests that the activity of bZIP19/23, through variations in the ZSM, can be modulated to impact Zn accumulation, which together with the evidence for an evolutionary conservation of the F-bZIP-regulated Zn deficiency response in land plants (Castro et al. 2017) constitutes a promising avenue for Zn biofortification in crops (Assunção 2022).
Cadmium (Cd) is one of the most toxic trace metals present in the environment, being released mainly from mining and industrial activities. In agricultural soils, contamination with Cd results from atmospheric deposition and application of phosphate fertilizers, manure and sewage sludge (McLaughlin et al. 2021). Cd is generally mobile in soil and taken up by plants, therefore the intake of plant-derived foods is considered the dominant source of Cd exposure in human population (Clemens et al. 2013a). Zn and Cd have similar chemical properties and may interact through competition for the same uptake membrane transporters (Chang et al. 2023). This raises the concern that strategies for Zn biofortification of crops will also increase Cd accumulation when grown on soil with Cd availability, which can be found in agricultural soils around the word (Clemens et al. 2013a).
Here, to advance knowledge of the effects of deregulation of the Zn deficiency response on plant growth and Zn accumulation, and to evaluate whether this deregulation influences accumulation of Cd, we analyse Arabidopsis mutant lines grown on soil with and without Cd availability. The Arabidopsis bzip19 bzip23 double mutant (bzip19/23) lacks a functional bZIP19 and bZIP23, and exhibits a hypersensitive phenotype when exposed to Zn deficiency stress, which is functionally complemented by constitutive expression of either bZIP19 or bZIP23 (Assunção et al. 2010). Functional complementation with the bZIP19 with the ZSM deleted, instead of the native bZIP19, results in increased Zn accumulation in leaves and seeds, the latter by 50%, in soil-grown plants (Lilay et al. 2021). In this study we use the bZIP19-ZSM deletion line, bzip19/23-bZIP19(del1 del2), to analyse growth and developmental traits in soil-grown plants and analyse element concentration in different above-ground plant tissues, comparing plants grown on standard peat soil and Cd-spiked soil. We also analyse the Zn distribution in seed sections using LA-ICP-MS. Finally, to verify whether mutations in the ZSM represent gain-of-function dominant mutations, we analyse the constitutive expression of bZIP19-ZSM deletion in wild-type, instead of bzip19/23, genetic background, i.e. Col-0 bZIP19(del1 del2).
Methods
Plant material and growth conditions
The Arabidopsis thaliana (Arabidopsis) genotypes used in this study were the wild-type accession Columbia (Col-0), the double T-DNA insertion mutant bzip19 bzip23 (bzip19/23), described in Assunção et al. (2010), the bzip19/23-bZIP19 and bzip19/23-bZIP19(del1 del2) lines, with bZIP19 or bZIP19(del1 del2) under the CaMV35S promoter transformed in the bzip19/23 mutant background, described in Lilay et al. (2019, 2021). The bZIP19(del1 del2) variant of bZIP19 has a deletion in the two Cys- and His-rich regions of the Zn Sensor Motif (ZSM) (Lilay et al. 2021). The Col-0-bZIP19 and Col-0-bZIP19(del1 del2) lines were generated similarly to the bzip19/23-bZIP19 and bzip19/23-bZIP19(del1 del2) lines; the constructs pCaMV35S:bZIP19-CFP-HA and pCaMV35S:bZIP19(del1 del2)-CFP-HA, respectively, were used for stable transformation of wild-type Arabidopsis Col-0, as described in Lilay et al. (2019). Transgenic plants were selected by Basta resistance and homozygous transgenic seeds (T3 generation) of three to four independent lines per construct, were selected. In this study, two independent lines (1 and 2) of bzip19/23-bZIP19, bzip19/23-bZIP19(del1 del2), Col-0-bZIP19 and Col-0-bZIP19(del1 del2) were analysed. The bzip19/23-bZIP19 line 1 has been previously described in Lilay et al (2019, 2021), as bzip19/23-OE19 and bzip19/23-bZIP19 line 1, respectively. Analysis of this line confirmed an increased transcript level of the overexpressed bZIP19 gene, and a western blot anti-HA, confirmed the expected protein molecular weight for the bZIP19-CFP-HA expressed protein (Lilay et al. 2019). The bzip19/23-bZIP19(del1 del2) lines 1 and 2 have been previously described in Lilay et al. (2021) and their increased transcript level of the overexpressed bZIP19 gene is shown in Fig. SI-1. All lines analysed here are T3 generation and homozygous for the T-DNA insertion, except bzip19/23-bZIP19 line 1, which in this study, was analysed as T4 generation from a T3 homozygous line.
Prior to sowing, seeds were surface sterilized by treatment with chlorine (Cl2) gas for three hours in a desiccator jar (~ 6 L). Gas was generated within the desiccator by mixing 50 mL bleach with 700 μL 36% HCl. Subsequently, seeds were stratified in 0.25% agarose in the dark at 4 °C for 2 to 5 days. Sterilized seeds were sown under sterile conditions in 120 mm square petri dishes containing half-strength Murashige and Skoog (MS) medium with 1.5% (w/v) sucrose, 0.5 g L−1 MES and 1.2% (w/v) phyto-agar (Duchefa) at pH 5.7. Seedlings were grown for 7 days in a climate chamber at 22–20 °C (day/night), 70% relative humidity, and under a 16 h/8 h day/night cycle with approximately 125 μmoles/m2/s white light, with the petri diches placed vertically. Seven-day-old seedlings were transplanted to individual 50 mL pots filled with either standard peat soil (control soil) or 1 mg/kg Cd supplemented substrate (+ Cd soil). The substrate consisted of 4 parts of peat soil mixed with 1 part vermiculite. Peat soil was previously sieved (test sieve, aperture size 8 mm, ISO 3310–1). Control and Cd supplemented substrates were soaked for 3 days in 1 Lkg−1 demineralized water, and in 1 Lkg−1 demineralized water with 2.74 mgL−1 Cd(NO3)2·4H2O, respectively. Pots were arranged in greenhouse trays containing a grid of 4 by 7 pots. In total 14 replicates of Col-0 and bzip19/23 were grown per treatment. For bzip19/23-bZIP19 and bzip19/23-bZIP19(del1 del2) genotypes, two independently transformed lines were analysed (line 1 and line 2) and 7 replicates were grown per independently transformed line and per treatment (control soil and Cd-supplemented soil). For Col-0-bZIP19 and Col-0-bZIP19(del1 del2) genotypes, two independently transformed lines were analysed (line 1 and line 2) and 7 replicates were grown per independently transformed line in control soil (Fig. SI-2). After transplantation to soil, plants were grown in the climate chamber described above until seed-setting and desiccation. Plants were top irrigated per individual plant as needed upon observation (every second day). Upon soil preparation and once a week, the water was supplemented with beneficial nematodes Entonem (from Koppert) and Gnatrol® (biological larvicide), according to suppliers’ instructions to counteract herbivory.
Measurement of plant growth and developmental traits
Routine measurements of plant traits relating to development and growth were performed every other day, starting on day 5 after the transplantation to soil of seven-day-old seedlings. Measurements were performed according to the framework established by Boyes et al. (2001). Measured traits included the maximum rosette diameter, shoot length from rosette to the tip of the most apical flower, number of open flowers, and the number of siliques. In addition, the days at which plants bolted (stem longer than 1 mm) and flowered (first flower opened, i.e., petals spreading open) were recorded. Finally, the number of rosette leaves was counted upon flowering. Measurements were performed until day 37 after seedling transplantation to soil, at which the rosette growth appeared to reach a plateau. After seed setting and desiccation, seeds were collected per plant and weighed to determine yield. Plants affected by herbivory were marked, and excluded from statistical analysis of the affected traits, i.e., rosette diameter and stem length.
Soil analysis
The soil carbon (C) and nitrogen (N) content was analysed in 100 mg of soil sample using an elemental CN analyzer (CNS Vario Macro cube, Elementar Analysensysteme, GmbH, Germany). The pH measurements were performed in 5 g of air-dry soil (< 2 mm) in 25 mL of 0.01 M CaCl2 solution, according to the ISO 10390 reference. The soil DTPA extraction was performed in soil samples, ca. 5 g, extracted with 10 mL of 0.005 M DTPA + 0.01 M CaCl2 + 0.1 M TEA, according to (Lindsay and Norvell 1978). Samples were shaken (end over end) for 2 h before centrifugation at 3500 rpm for 10 min and 0.22 µm filtration of the supernatant. Samples were diluted using 2% TAG HNO3 immediately prior to ionomic analysis. For the C and N content, pH analysis and DTPA extraction, three soil sample replicas of each soil (control or Cd-supplemented) were analysed, to confirm that soils remained similar (Table SI-1).
Ionomic analysis
Forty-two days after seedling transplantation to soil, plant material was collected for elemental analysis by Inductively-Coupled-Plasma Mass Spectrometry (ICP-MS) and Inductively-Coupled-Plasma Optical Emission Spectrometry (ICP-OES). Three rosette leaves, four cauline leaves and four siliques were collected per plant. To prevent contamination, collection was done using plastic tweezers and samples were rinsed three times in ultrapure MilliQ water (Merck). Samples were collected in 1.5 mL microcentrifuge tubes and dried at 70 °C for two days. Four to seven replicates of rosette leaves, cauline leaves, siliques and seeds were analysed. Approximately 5 to 10 mg of plant tissue and 20 to 25 mg of seeds were weighed and used for elemental analysis.
Rosette leaves and seeds were digested in a pressurized microwave oven (Ultrawave, Milestone Inc.) using ultra-pure acids (70% HNO3, 15% H2O2) at 240°C and 8000 kPa for 15 min. Prior to elemental analysis, samples were diluted to a concentration of 3% HNO3. For Cd, elemental analysis by ICP-MS was performed using an Agilent 8900 equipped with SeaSpray nebulizer and Scott double-pass spray chamber (Agilent Technologies). Samples were automatically introduced by an Agilent integrated autosampler with 89 positions. Sample introduction was done from 15 mL falcon tubes using an ASX-530 CETAC automatic sampler. For every 20 samples, a certified reference (NIST1515, Apple leaf, National Institute of Standards and Technology, USA) was included in the ICP-MS analyses to assess measurement quality and perform drift correction. For all other elements, elemental analysis by ICP-OES was performed using an inductively coupled plasma optical emission spectrometer (5100 ICP-OES, Agilent Technologies). The ICP-OES was equipped with a SeaSpray nebulizer and a double-pass Scott-type spray chamber. Automatic sample introduction was performed from a ASX-520, CETAC auto-sampler from 15 ml falcon tubes. Certified reference material (NIST1515, apple leaf, National Institute of Standards and Technology) was included to evaluate the data quality and drift correction was performed on the basis of drift samples for every 20 samples. These analyses were done at CHEMI Center, University of Copenhagen. ICP-MS and ICP-OES data were processed using the Agilent ICP Masshunter Software (version 4.3) and Agilent ICP Expert Software (version 7.3).
Soil samples, cauline leaves and siliques were digested in 1 mL concentrated trace analysis grade (TAG) HNO3 acid at 115˚ C for 4 h, and ICP-MS was performed for all elements, at the ICP-MS Analysis Facility (School of Biosciences, University of Nottingham). Prior to elemental analysis, samples were diluted to 10 mL with 18.2 MΩ Milli-Q water (Merck Millipore). ICP-MS was performed using a Thermo-Fisher Scientific iCAP-Q (Thermo Fisher Scientific, Bremen, Germany). Samples were introduced at a flow rate of 1.2 mL min−1 from an autosampler (Cetac ASX-520) incorporating an ASXpress™ rapid uptake module through a perfluoroalkoxy (PFA) Microflow PFA-ST nebuliser (Thermo Fisher Scientific, Bremen, Germany). Analysis used kinetic energy discrimination (KED) and a He charged collision cell to remove polyatomic interferences. Internal standards Sc (10 µg L−1), Ge (10 µg L−1), Rh (5 µg L−1) and Ir (5 µg L−1) were used to correct for instrumental drift, and introduced to the sample stream on a separate line (at equal flow rate) via the ASXpress unit. The matrices used for internal standards, calibration standards and sample diluents were 2% TAG grade HNO3 (Fisher Scientific, UK) with 4% methanol to enhance ionization of some elements. Sample processing was undertaken using Qtegra™ software (Thermo-Fisher Scientific) utilizing external cross-calibration between pulse-counting and analogue detector modes when required.
LA-ICP-MS analysis of seeds
Seeds of the genotypes wild-type Col-0, bzip19/23 double mutant, bzip19/23-bZIP19 and bzip19/23-bZIP19(del1 del2) were embedded in Optimal Cutting Temperature (OCT) medium (Tissue-Tek) and were frozen at -25 °C. Frozen blocks were transferred to a Leica CM3050 S cryo-microtome and were sectioned into 16 µm thick slices. Prior to each cut a piece of Cryofilm type 2C (Section-lab Co. Ltd., Yokohama, Japan) was adhered to the surface that was about to be cut, allowing the samples to stay intact and able to be handled after cutting. Sections were inspected by light microscopy to identify seeds that were oriented such that cutting produced transvere sections of the embryo. Once identified, subsequent samples were adhered to microscope slides using double sided tape. Samples were freeze dried for at least two days, after which they were stored in a protective card box at room temperature. Seed sections selected for further analysis were imaged using a Leica DM2000 LED light microscope equipped with Flexacam C1 digital camera, using a 20X magnification objective. Bioimaging of seed sections was performed using Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS) equipped with a 193 nm Argon Fluoride (ArF) excimer laser and cobalt cell (Iridia, Teledyne Photon Machines, Thousand Oaks, CA 91360, USA). Elemental signals were measured using an Agilent 8900 ICP-MS. Laser ablation was done with a scan speed of 160 μm s−1, repetition rate of 200 Hz, spot size of 4 µm (circles), and an energy fluence of 0.5 J cm−2. The laser ablation unit was connected to the ICP-MS using Helium (He) as transfer gas, with a flow rate of 0.15 mL min−1. In addition, the ICP-MS was configured using the standard mode, with a 30 mm sample cone depth, and Argon (Ar) nebulizer gas with a flow rate of 0.75 mL min−1. Scanning by ICP-MS was done for 39 K, 55Mn, 56Fe, and 66Zn using a 25 ms scan cycle with integration times of 0.5, 2.0, 4.5, and 10 ms, respectively. Before and after each analysis, a NISU612 glass standard was used to monitor any drift in sensitivity (NIST; National Institute for Standards and Technology, SC, USA). Three to six replicate of seed transverse sections were imaged per genotype. Data was processed using HighDefinition Imaging Processing (HDIP) software (Teledyne CETAC Technologies), including gas blanks and drift corrections.
Statistical analysis
Data was analysed using the statistical software R (version 4.1.3). To investigate the effect of plant genotypes and soil treatments on the traits bolting day, flowering day, yield, and number of rosette leaves upon flowering, and on the ionomics output, data was analysed using analysis of variance (ANOVA) tests, and post-hoc pairwise comparisons using Tukey’s range test. To investigate the effect on stem length and rosette diameter over time, data were analysed using mixed ANOVA tests, and post-hoc pairwise testing using Tukey’s range test.
Results
Soil-grown Arabidopsis lines overexpressing bZIP19 with the Zn sensor motif (ZSM) deleted do not show developmental penalties
A modified bZIP19 protein, with the Cys/His-rich regions within the ZSM deleted, bZIP19(del1 del2), and its transcriptional expression under a constitutive CaMV35S promoter in the Arabidopsis bzip19/23 double mutant, was previously investigated (Lilay et al. 2021). To further examine bzip19/23-bZIP19(del1 del2) and assess the effect of the bZIP19 ZSM deletion and deregulation of the Zn deficiency response, we analysed plant growth and developmental traits in bzip19/23-bZIP19(del1 del2), in bzip19/23-bZIP19 with constitutive expression of bZIP19 native gene, in bzip19/23 double mutant and in Col-0 wild-type. Seven-day-old seedlings, germinated on MS medium, were transplanted to a standard peat soil (control) and a Cd-supplemented soil (+ Cd) and grown until seed setting. The mean values of Cd concentration reported for agricultural top soils around the world are in the range of 0.2–1.0 mg Cd Kg−1 dry soil (Clemens et al. 2013a), with a remark for the enormous variation and much higher values that can be found. The target value of 0.8–1.0 mg Cd Kg−1 soil, at which further investigation of soil contamination is warranted, is established in some European countries, such as The Netherlands and Finland (Robberse and Denneman 1993; Ministry of the Environment, Finland 2007). To mimic a soil containing Cd close to that range, the standard peat soil used as Control was spiked with 1 mg Cd per kg soil. Therefore, two soil treatments were tested: control soil and Cd-spiked soil.
Analyses of plant growth and developmental traits included measuring the following parameters (Boyes et al. 2001); average number of rosette leaves upon flowering, average yield (mg), average bolting and flowering time (days after transplantation to soil), and average rosette diameter and stem length (mm) over time (Fig. 1A-H). Data obtained for bolting and flowering day, number of rosettes upon flowering and seed yield showed no consistent significant differences between the genotypes and the soil treatments, despite some variation between the lines (Fig. 1A-D). The bzip19/23-bZIP19(del1 del2) line 2 showed a small but significant difference between control and Cd-supplemented soil in flowering day and number of rosette leaves upon flowering, though it was not consistent between the two independent lines (line 1 and 2). While the bzip19/23-bZIP19 line 1 showed a significant difference between soil treatments in seed yield, it was within the variation observed in the other lines. Data obtained for rosette diameter and stem length over time after transplantation to soil also showed no consistent differences between genotypes and soil treatments (Fig. 1E-H). The bzip19/23-bZIP19(del1 del2) line 2 in Cd-supplemented soil showed a delayed bolting and flowering day, and a higher number of rosette leaves upon flowering (Fig. 1 ABFH). This agrees with our observation that line 2 had delayed seed germination. Nonetheless, its plant growth pattern was comparable with that of the other line and genotypes. Although the traits we analysed showed some variation between lines, results show no consistent differences in the growth and development parameters between wild-type, bzip19/23 double mutant, and the complementation lines bzip19/23-bZIP19 and bzip19/23-bZIP19(del1 del2), grown on control or Cd-supplemented soils (Fig. 1), therefore suggesting that the bzip19/23 double knock-out or the bZI19 or bZIP19(del1 del2) overexpression do not noticeably impact plant growth and development.
Plant growth and development analysis of bzip19/23-bZIP19(del1 del2) lines. The Arabidopsis Col-0 wild-type, bzip19/23 double mutant, bzip19/23-bZIP19 and bzip19/23-bZIP19(del1 del2) lines were grown in peat soil (control soil) or in peat soil spiked 1 mg Cd per kg soil (+ Cd soil). The graphs show (A) average bolting time, (B) average flowering time, (C) average number of rosette leaves upon flowering, (D) average yield (mg), (E) average rosette diameter over time on control soil and (F) Cd-spiked soil, and (G) stem length (mm) over time on control soil and (H) Cd-spiked soil. Seeds were germinated on plates with MS medium and 7-day-old seedlings were transferred to soil. Time is measured as days after transplantation of seedlings to soil. The bzip19/23-bZIP19 and bzip19/23-bZIP19(del1 del2) lines are homozygous T3 progeny of two independently transformed lines designated 1 and 2. Data represent average ± SEM, n = 7–14 (A-D) and n = 4–14 (E–H). Letters indicate significance groups determined by ANOVA followed by Tukey pairwise comparison (P < 0.05)
To investigate the effect of the ZSM deletion in bZIP19 in a Col-0 genetic background and assess whether it is a dominant mutation, bZIP19(del1 del2) and bZIP19 were transcriptionally expressed with a constitutive CaMV35S promoter in Arabidopsis Col-0 wild-type. The genotypes Col-0 bZIP19(del1 del2), Col-0-bZIP19 and Col-0 wild-type were analysed in the same manner as the lines in the bzip19/23 background. Data from growth and development parameters showed, overall, no consistent significant differences between the genotypes, (Fig. 2A-F) similarly to the results obtained with the bzip19/23 background lines (Fig. 1). Col-0-bZIP19(del1 del2) line 2 bolted and flowered slightly but significantly later than the wild-type, however this was not the case for line 1 (Fig. 2A,B). The number of rosette leaves and seed yield in wild-type was significantly different from Col-0-bZIP19 line 2 and, for seed yield, from Col-0-bZIP19(del1 del2) line 2 as well, but the other independent line (line 1) of both genotypes had intermediate values (Fig. 2C,D). Also here, in the analysis with the Col-0 genetic background, although with some variation in the traits analysed between the lines, results indicate that there are no consistent differences in the growth and development parameters between wild-type, Col-0-bZIP19 and Col-0-bZIP19(del1 del2) lines (Fig. 2).
Plant growth and development analysis of Col-0-bZIP19(del1 del2) lines. The Arabidopsis Col-0, Col-0-bZIP19 and Col-0-bZIP19(del1 del2) lines were grown in peat soil (Control soil) and Col-0-bZIP19 and Col-0-bZIP19(del1 del2) are constitutively expressing bZIP19 or bZIP19(del1 del2) in Arabidopsis wild-type background (Col-0 background). The graphs show (A) average bolting time, (B) average flowering time, (C) average number of rosette leaves upon flowering, (D) average yield (mg), (E) average rosette diameter over time, and (F) stem length (mm) over time. Seeds were germinated on plates with MS medium and 7-day-old seedlings were transferred to soil. Time is measured as days after transplantation of seedlings to soil. The Col-0-bZIP19 and Col-0-bZIP19(del1 del2) lines are homozygous T3 progeny of two independently transformed lines designated 1 and 2. Data represent average ± SEM, n = 7–14 (A-D) and n = 4–14 (E,F). Letters indicate significance groups determined by ANOVA and Tukey pairwise comparison (P < 0.05)
Soil-grown bzip19/23-bZIP19(del1 del2) plants have an increased and specific Zn accumulation even on Cd-spiked soil
Forty-two days after seedling transplantation to soil, or after seed desiccation, plant material was collected for ionomic analysis. Rosette and cauline leaves, siliques, and seeds were collected from the plants grown on control soil and Cd-spiked soil. Among the plants grown on control soil, the bzip19/23-bZIP19(del1 del2) lines showed a significantly higher Zn concentration in seeds (ca. 1.5-fold), siliques (ca. 1.5-fold), and rosette (ca. 2.5-fold) and cauline (ca. 3.0-fold) leaves compared with the wild-type, bzip19/23 and bzip19/23-bZIP19 lines (Fig. 3A-D). Between these three genotypes, there were no consistent significant differences in Zn concentration, except in seeds of bzip19/23 mutant which had a significantly lower Zn concentration than the wild-type seeds. Comparing the Zn concentration in seeds, siliques, and rosette and cauline leaves from each genotype between control soil and + Cd soil, there were no significant differences, except in rosette and cauline leaves of bzip19/23-bZIP19(del1 del2) line 1, but not line 2, and in siliques of bzip19/23-bZIP19 line 1, but not line 2, which showed higher Zn content in + Cd soil (Fig. 3CD). These results showed a significant increase in Zn concentration in all plant tissues analysed in bzip19/23-bZIP19(del1 del2) lines compared with the other genotypes, and the increase was similar between bzip19/23-bZIP19(del1 del2) plants grown on control or Cd-spiked soil.
Ionomic analysis of bzip19/23-bZIP19(del1 del2) lines. The Arabidopsis Col-0 wild-type, bzip19/23 double mutant, bzip19/23-bZIP19 and bzip19/23-bZIP19(del1 del2) lines were grown in peat soil (control soil) or in peat soil spiked 1 mg Cd per kg soil (+ Cd soil). The graphs show element concentration (µg g−1 dw) in seeds, siliques, rosette leaves, and cauline leaves of (A-D) Zn, (E–H) Cd, (I-L) Fe, (M-P) Mn, (Q-T) Cu, and (U-X) P. The bzip19/23-bZIP19 and bzip19/23-bZIP19(del1 del2) lines are homozygous T3 progeny of two independently transformed lines designated 1 and 2. Leaves were collected 42 days after transplantation of 7-day-old seedlings to soil, and seeds were collected after desiccation. Data points are average ± SEM, n = 7, except for Col-0 with n = 14. Letters indicate significance groups determined by ANOVA and Tukey pairwise comparison (P < 0.05)
The Cd concentration in seeds, siliques, and leaves of plants grown on control soil was residual (< 0.26 µgg−1 dry weight in seeds and siliques, and < 1.47 µgg−1 dry weight in leaves), while in plants grown on Cd-spiked soil, the concentrations ranged between 4.42–9.25 µgg−1 dry weight in seeds and siliques, and 36.95–74.12 µgg−1 dry weight in rosette and cauline leaves (Fig. 3E-H). The Cd concentrations of these tissues in plants grown in + Cd soil were not significantly different between genotypes, except in cauline leaves where slightly more Cd accumulated in bzip19/23-bZIP19(del1 del2).
Analysis of DTPA-extractable soil elements in the control and Cd-spiked soils showed that only Cd, among the other analysed elements, significantly differed in concentration between the soils; 0.07 ± 0.01 and 2.44 ± 0.20 mg kg−1 in control and + Cd, respectively (Table SI-2). The concentration of extractable Cd in the + Cd soil was higher than the concentration of CdSO4 added to the soil, which was 1 mg of Cd per kg soil. This difference is likely caused by the evaporation of moisture from the soil, considering that the Cd was supplemented into non-dried standard peat soil whereas the soil analysis was performed on fully dried soil. Together, these results showed that the Cd accumulation in seeds, siliques and rosette leaves was not significantly different between all genotypes, thus with no differences between bzip19/23-bZIP19(del1 del2) and wild-type.
Ionomic data for other elements, namely Fe, Mn, Cu, P (Fig. 3I-X), B, Mg and Ca (Fig. SI-3) showed that there were no major variations in the concentrations of these elements in leaves, siliques and seeds between wild-type, bzip19/23, bzip19/23-bZIP19 and bzip19/23-bZIP19(del1 del2). The concentration of Fe, Mn, Cu and P did not show consistent significant differences between the genotypes. The concentration of Fe and Mn in bzip19/23-bZIP19(del1 del2) seeds was slightly higher than in the wild-type seeds but only in one of the two independent lines (Fig. 3IM). The element concentration data between plants grown on control soil and Cd-spiked soil from the same genotype did not show overall major variations (Fig. 3I-X). Nonetheless, they indicate an interaction between Cd and Mn, with higher Mn concentration observed in rosette and cauline leaves, and to a lesser extent in seeds, in plants grown on + Cd soil (Fig. 3M-P). An indication of interaction with Cd was also observed for Cu and Fe, with slightly higher Cu concentration in seeds from the + Cd soil (Fig. 3Q), whereas the effect seemed to be the opposite in rosette leaves (Fig. 3S), while for Fe, its concentration in leaves from + Cd soil decreases in wild-type, bzip19/23 and bzip19/23-bZIP19 but not in bzip19/23-bZIP19(del1 del2) (Fig. 3K). These results indicate that the concentration of other elements, namely Fe, Mn, Cu and P, in leaves, siliques and seeds do not show major differences between genotypes, supporting that the increased Zn concentration in bzip19/23-bZIP19(del1 del2) is largely Zn-specific. In addition, the comparison between control and + Cd soil per genotype highlighted putative interactions between Cd and Mn, Fe or Cu.
Col-0-bZIP19(del1 del2) and bzip19/23-bZIP19(del1 del2) plants have similar Zn accumulation patterns
Analysis of element concentration in seeds, siliques and leaves was also performed in Arabidopsis lines with Col-0 genetic background. In all tissues, the Zn concentration was significantly higher in Col-0-bZIP19(del 1del2) lines than in Col-0-bZIP19 and wild-type, with an increase about 2.5-fold for rosette and cauline leaves, and up to 1.5-fold for seeds and siliques (Fig. 4A-D). These results showed a similar increase in Zn concentration in leaves, siliques, and seeds of Col-0-bZIP19(del1 del2) and bzip19/23-bZIP19(del1 del2) plants in comparison with the wild-type (Fig. 3A-D, 4A-D). Seeds from both genotypes displayed a ca. 50% increase in Zn content compared with wild-type seeds. The ionomic data for other elements, namely Fe, Mn, Cu, P (Fig. 4E-T), B, Mg and Ca (Fig. SI-4) showed no major variations and no consistent significant differences between Col-0-bZIP19(del1 del2), Col-0-bZIP19, and wild-type for each of the tissue analysed, similarly to the lines in the bzip19/23 background (Fig. 3I-X) (Fig. SI-3). These results showed that the element concentration patterns in leaves and seeds of Col-0-bZIP19(del1 del2) is comparable to that of bzip19/23-bZIP19(del1 del2), with an increased accumulation of Zn in both genotypes. These comparable ionomic results between lines with bzip19/23 or Col-0 genetic backgrounds supports that the bZIP19-ZSM deletion represents a gain-of-function dominant mutation.
Ionomic analysis of Col-0-bZIP19(del1 del2) lines. The Arabidopsis Col-0 wild-type, Col-0-bZIP19 and Col-0-bZIP19(del1 del2) lines were grown in peat (control) soil. The graphs show element concentration (µg g−1 dw) in seeds and leaves of (A-D) Zn, (E–H) Fe, (I-L) Mn, (M-P) Cu, and (Q-T) P. The Col-0-bZIP19 and Col-0-bZIP19(del1 del2) lines are homozygous T3 progeny of two independently transformed lines designated 1 and 2. Leaves were collected 42 days after transplantation of 7-day-old seedlings to soil, and seeds were collected after desiccation. Data points are average ± SEM, n = 7, except for Col-0 with n = 14. Letters indicate significance groups determined by ANOVA and Tukey pairwise comparison (P < 0.05)
LA-ICP-MS shows Zn localization in bzip19/23-bZIP19(del1 del2) seeds mainly in the embryo
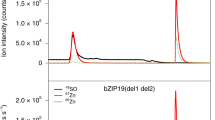
To gain insight into the Zn spatial distribution in bzip19/23-bZIP19(del1 del2) seeds, transverse seed sections were analysed by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). Seeds of wild-type, bzip19/23, bzip19/23-bZIP19 and bzip19/23-bZIP19(del1 del2) were analysed to obtain spatially resolved images of Zn, Fe and Mn distribution. Using bright field microscopy, structures of mature seeds can be identified, including the cotyledons and the hypocotyl and radicle axis and the seed coat. These images do not show visible morphological differences between the genotypes (Fig. 5A). The spatial distribution of Zn, Fe, and Mn obtained with the LA-ICP-MS analysis in wild-type seeds showed Zn distributed evenly throughout the embryo, Fe accumulated around the provasculature, and Mn present in the regions adjacent to the provasculature, mainly in the cotyledons (Fig. 5B). Spatial distribution of Zn, Fe, and Mn in seeds from bzip19/23, bzip19/23-bZIP19 and bzip19/23-bZIP19(del1 del2) did not show major differences from what was observed in the wild-type. In line with this, Zn was found mainly in the embryo and not in the seed coat in seeds from bzip19/23-bZIP19(del1 del2). The observed higher Zn signal intensity in bzip19/23-bZIP19(del1 del2) seeds, supports its increased Zn accumulation (Figs. 3 and 5B).
Zn distribution in seeds from bzip19/23-bZIP19(del1 del2). A) Bright field image of transverse sections of seeds from Arabidopsis wild-type Col-0, bzip19/23 double mutant, bzip19/23-bZIP19 (line 2) and bzip19/23-bZIP19(del1 del2) (line 2). Letters refer to the two cotyledons, C, and to the hypocotyl and radicle axis, H, from the mature embryo, and to the seed coat, SC. B) Element profiles of transverse sections of seeds from the four genotypes showing spatial distribution of Zn (66Zn), Fe (56Fe) and Mn (55Mn) measured by LA-ICP-MS, n = 6 for Col-0 and bzip19/23-bZIP19(del1 del2) and n = 3 for bzip19/23 and bzip19/23-bZIP19, scale bar = 200 µm. Seed sections were 16 µm thick and the ablation area diameter was 4 µm
Discussion
Deregulation of the Zn deficiency response in Arabidopsis by deletion of the bZIP19-ZSM does not appear to cause developmental penalties
It was previously shown that ZSM mutations, such deletions of the two Cys/His-rich regions or even substitutions of Cys/His residues, affect the Zn sensor function and consequently the activity of bZIP19/23 transcription factors. Thereby leading to a Zn-insensitive and constitutive activation of target gene expression, including ZIP and NAS genes (Lilay et al. 2021) (Fig. SI-5). Considering that bZIP19 and bZIP23 are the central regulators of the Zn deficiency response in Arabidopsis, mutations in the ZSM cause a deregulation of this response. The effect of such deregulation on plant performance has not been investigated in detail.
To gain knowledge on the effect that mutations in the ZSM of the bZIP19 transcription factor have on plant development and the plant ionome, soil-grown Arabidopsis lines constitutively expressing the native bZIP19 or the ZSM-deletion mutant bZIP19(del1 del2) (Lilay et al. 2021) were analysed. Several growth and developmental traits were monitored, including the number of rosette leaves upon flowering, bolting and flowering time, seed yield, rosette diameter, and stem length. Each trait was measured in wild-type Col-0, the bzip19/23 double mutant, and the complementation lines bzip19/23-bZIP19 and bzip19/23-bZIP19(del1 del2). Overall, no major differences were measured between genotypes across all parameters analysed, both when plants were grown on standard peat control soil or on Cd-spiked soil (Fig. 1). Bright-field microscope images of mature seed sections of Col-0, bzip19/23, bzip19/23-bZIP19, and bzip19/23-bZIP19(del1 del2) showed comparable seed size and structures between all genotypes (Fig. 5A). These results indicate that bzip19/23-bZIP19(del1 del2) plants with the bZIP19-ZSM deletion do not exhibit visible growth and developmental phenotypes. Moreover, it indicates that the lack of a functional bZIP19 and bZIP23 protein, or constitutive expression of bZIP19, as in the bzip19/23 double mutant and bzip19/23-bZIP19 complementation line, respectively, do not appear to have an impact on growth and development (Fig. 1). These findings support that bZIP19 and bZIP23 have a highly specific function in the Zn deficiency response (Assunção 2022), and that the deregulation of the Zn deficiency response in bZIP19-ZSM deletion lines does not appear to cause penalties in plant growth, development, or reproduction.
Deregulation of the Zn deficiency response in bZIP19-ZSM deletion leads to Zn accumulation in leaves and seeds that is largely Zn-specific
In addition to developmental traits, the plant ionome was analysed in different plant tissues. Compared with wild-type and bzip19/23-bZIP19 plants, bzip19/23-bZIP19(del1 del2) plants had significantly increased Zn concentrations in rosette and cauline leaves (ca. 2.5-fold), and siliques and seeds (ca. 1.5-fold) (Fig. 3) in agreement with previous observations on the same genotypes (Lilay et al. 2021). LA-ICP-MS analysis of transverse seed sections indicated that, in bzip19/23-bZIP19(del1 del2), Zn is distributed mainly throughout the embryo, and not in the seed coat, similarly to what was observed, and is reported, in the wild-type (Fig. 5CD; Kim et al. 2006; Lee et al. 2021). Moreover, the higher Zn signal intensity in bzip19/23-bZIP19(del1 del2) compared to wild-type seeds, supports its increased Zn concentration.
Deletion of the ZSM of bZIP19 in the bzip19/23-bZIP19(del1 del2) genotype leads to a Zn-insensitive and constitutive expression of bZIP19/23 target genes, and thus a deregulation of the Zn deficiency response (Lilay et al. 2021). Target genes encode ZIP-family transporters, which mediate cellular Zn uptake, and NAS enzymes that produce nicotianamine, a Zn ligand involved in its intercellular movement and distribution (Guerinot 2000; Clemens et al. 2013b). The function of individual ZIP transporters in plant Zn homeostasis is generally not well understood, likely in part due to functional redundancies (Lee et al. 2021). Further research into the localization and specificity and affinity properties of ZIPs, in particular those regulated by bZIP19/23, will help in better understanding their role in Zn nutrition and their contribution to the Zn accumulation phenotype of bZIP19-ZSM deletion lines. Recently, element distributions within seeds of irt3 zip4 zip6 zip9 quadruple loss of function Arabidopsis mutants were quantified using synchrotron-based X-ray fluorescence (SXRF), with Zn showing an even distribution throughout the embryo, but at a lower concentration compared to wild-type embryos (Lee et al. 2021). Furthermore, only triple or quadruple ZIP mutants showed Zn related phenotypes, supporting functional redundancy (Lee et al. 2021). The genes encoding IRT3, ZIP4, and ZIP9 are targets of bZIP19/23 transcription factors, and mediation of Zn transport was shown for IRT3 and ZIP4 (Lin et al. 2009; Assunção et al. 2010). The reported Zn distribution and accumulation in quadruple mutant seed is comparable to what was found here in the bzip19/23 mutant (Fig. 5B), and altogether, this supports the importance of ZIP transporters in the bZIP19/23 regulated Zn deficiency response.
Although bzip19/23 mutant plants do not appear to have developmental penalties when grown on soil (Fig. 1), the lack of a functional bZIP19/23-regulated Zn deficiency response still appears to reduce seed Zn content (Figs. 3 and 5B), underpinning the importance of this regulatory mechanism in coping with fluctuations of Zn availability in the soil. Across the route that Zn takes through the plant and delivery into seeds there are several apoplastic barriers to be crossed. In Arabidopsis the P1B-type Heavy Metal ATPases HMA2 and HMA4 play an important role in this regard. They are plasma membrane transporters that pump Zn, and also Cd, from the cytosol into the apoplast. In addition to being involved in Zn export and xylem loading in roots (Hussain et al. 2004), HMA2 and HMA4 are required for the transfer of Zn from the maternally derived seed coat tissue, into the endosperm and embryo filial tissue (Olsen et al. 2016). Analyses of Zn distribution and quantification in seeds of the hma2hma4 double mutant have shown that plants lacking both these Zn pumps accumulate much less Zn in the embryo and instead have Zn accumulation in the seed coat. This suggested that cellular export of Zn towards the seed filial parts is blocked without the function of HMA2 and HMA4 (Olsen et al. 2016). Here, Zn is mostly present throughout the embryo and not in the seed coat in bzip19/23-bZIP19(del1 del2) seeds (Fig. 5B). The expression of HMA2 and HMA4 is not responsive to Zn-deficiency (Sinclair and Krämer 2012) and, as per current knowledge, they are not target genes of bZIP19/23 transcription factors. Thus, in bzip19/23-bZIP19(del1 del2), which has a deregulated Zn deficiency response, the normal delivery of Zn into the seed embryo is not disrupted and appears to be maintained by the regular combined activity of HMA2 and HMA4 transporters.
The ionomic data showed that, with the exception of Zn, the concentration of Fe, Cu, Mn and P in leaves, siliques and seeds did not show consistent significant differences between wild-type, bzip19/23, bzip19/23-bZIP19 and bzip19/23-bZIP19(del1 del2) (Fig. 3). In bzip19/23-bZIP19(del1 del2) lines, there was a slight, but not consistent nor significant, increase of Fe in seeds, also observed in Lilay et al (2021). The Fe and Mn distribution patterns obtained from the seed LA-ICP-MS analysis were comparable between the different genotypes (Fig. 5B) and their distributions were in line with previous reports. Namely, Fe is reported in endodermal cells surrounding embryo’s provascular tissue, and Mn accumulates in cortical cells of the hypocotyl and in subepidermal cells at the abaxial side of cotyledons (Kim et al. 2006; Eroglu et al. 2017; Lee et al. 2021). Concerning P, its concentration in seeds was not significantly different between genotypes. Most P in seeds is stored as phytate, which is negatively charged and therefore forms mixed salts with metal cations including Zn and Fe. These metal-phytate complexes are poorly soluble and therefore reduce the bioavailability of Zn and Fe from crop grains for intestinal absorption (Bentsink et al. 2003). In Arabidopsis mature seeds, most Zn is present in the embryo as Zn-phytate complexes (Otegui et al. 2002). Here, while bzip19/23-bZIP19(del1 del2) seeds showed a ca. 50% increase in Zn concentration compared to wild-type, they did not show an increase in P concentration. The implication of this is that the additional Zn might be more available for intestinal absorption, if not all is bound in metal-phytate complexes.
Altogether, these results showed that deregulation of the Zn deficiency response in bZIP19-ZSM deletion lines leads to an increased and largely Zn-specific accumulation in leaves and seeds, the latter by 50%, and that the Zn is distributed throughout the seed embryo.
Exposure to low-level Cd contamination does not cause increased Cd accumulation in bZIP19-ZSM deletion compared with wild-type
The increased Zn accumulation in leaves and seeds from soil-grown bzip19/23-bZIP19(del1 del2) plants could raise the concern that, in soils with available Cd, there would be increased Cd accumulation compared with the wild-type plants. This was investigated by comparing plants grown in a control soil and in Cd-spiked soil, the latter with 2.44 mg Kg−1 of extractable Cd (Table SI-2). Plants grown in Cd-spiked soil showed an increase in Cd accumulation in leaves, siliques and seeds compared with the plants grown in control soil, which had showed residual Cd concentration in these organs. However, there were no consistent significant differences in Cd concentration between the genotypes (Fig. 3E-H). Considering that there were also no major differences in Zn accumulation between bzip19/23-bZIP19(del1 del2) plants grown on control or + Cd soils (Fig. 1), these results indicate that the increased Zn accumulation in soil-grown plants of a bZIP19-ZSM deletion line with a deregulated Zn deficiency response is not affected by the low-level of Cd exposure used in our experiment. Cd is a highly toxic element and in plants it is taken up by membrane transporters of the essential micronutrients Fe, Mn and Zn. In Arabidopsis, the Fe transporter IRT1 (Iron-Regulated Transporter 1) and manganese (Mn) transporter NRAMP1 (Natural Resistance- Associated Macrophage Protein 1) mediate the uptake of a broad range of divalent cations including Fe2+, Mn2+, Zn2+ and Cd2+ (Thomine et al. 2000; Vert et al. 2002; Cailliatte et al. 2010). Cd may interact with these micronutrients through competition for the same membrane transporters, although factors such as availability in the soil, concentration ratios between elements, and transporter affinities will also play a role. HMA3, another P1B-type Heavy Metal ATPases member, is a tonoplast transporter that effluxes Zn and Cd from the cytosol into the vacuole, and is involved in sequestration of these and other toxic metals, in roots. Interestingly, the Arabidopsis Col-0 accession has a HMA3 loss-of-function allele and this accession has higher Cd concentration in leaves compared with Arabidopsis accessions with a functional HMA3 allele, indicating that the sequestration of Cd in the root vacuole affects its translocation to the shoot (Morel et al. 2009; Chao et al. 2012). Both accessions, with a non- and functional HMA3 allele, have similar Zn concentration in leaves, and complementation analysis indicated that, with respect to Zn, the homeostasis network regulation, involving the Zn deficiency response, counterbalances the inter-accessions genetic variation in the function of HMA3 (Pita-Barbosa et al. 2019). Our study was performed with the Col-0 accession and indicates that, although with a decreased capacity for Cd vacuolar sequestration in the root, the deregulation of the Zn deficiency response did not lead to an increase in shoot Cd accumulation, as inferred from the Cd concentration in leaves, siliques and seeds (Fig. 1A-D). There was, nonetheless, a small but significantly higher Cd concertation in cauline leaves of bzip19/23-bZIP19(del1 del2) compared with the wild-type. Overall, our results suggest that in the bZIP19-ZSM deletion lines, exposed to low-level Cd contamination, the constitutive expression of target genes, including ZIP transporter genes, does not contribute to increased Cd uptake and accumulation in the shoot. Regarding other elements, we did find evidence for putative interactions between Cd availability and the accumulation of other elements, namely Mn and Fe. The Mn concentration in leaves, and to a lesser extent in siliques and seeds, was higher in plants from all genotypes grown in Cd-supplemented soil compared to those in control soil (Fig. 1M-P). The concentration of Fe in leaves decreases in plants exposed to Cd, except in the bzip19/23-bZIP19(del1 del2) genotype, which could indicate some interaction between Fe uptake and the deregulation of the Zn deficiency response (Fig. 1I-L).
These results showed that exposure of bZIP19-ZSM deletion lines to low-level Cd contaminated soil does not cause increased Cd accumulation in leaves and seeds, compared with the wild-type, nor affects its Zn accumulation levels. This is relevant because Cd uptake is a concern in Zn biofortification efforts in crops. There are nonetheless species-specificities in Cd uptake and distribution, namely in monocot crops such as rice (Chen et al. 2018; Chang et al. 2023), that need to be considered and investigated before generalizing this conclusion to crop species.
Deletion of the ZSM from bZIP19 results in a gain-of-function dominant mutation
The Zn accumulation phenotype of bzip19/23-bZIP19(del1 del2) plants derives from the constitutive expression of bZIP19-ZSM deletion. To investigate the effect of bZIP19-ZSM deletion overexpression in the wild-type genetic background, which has endogenous expression of bZIP19 and bZIP23 unlike the bzip19/23 double mutant, the lines Col-0-bZIP19(del1 del2) were produced and analysed. The same plant developmental traits and ionomic analysis (Fig. 4) were performed in the Col-0 background lines, i.e. Col-0-bZIP19(del1 del2) and, as controls, Col-0-bZIP19 and Col-0. There were no apparent growth or developmental penalties, similarly to the bzip19/23 background lines (Figs. 1 and 2), and the ionomic results were also comparable (Fig. 3 and 4). The Col-0-bZIP19(del1 del2) plants had an increased Zn concentration in leaves (ca. 2.5-fold) and seeds (ca. 1.5-fold) similar to that of bzip19/23-bZIP19(del1 del2), and also largely Zn-specific (Fig. 4A). Deletion of the ZSM should prevent the binding of Zn2+ ions to this sensor motif and causes bZIP19 protein to become insensitive and no longer regulated by the cellular Zn status. As a consequence, transcriptional target gene activation becomes constitutive leading to a Zn accumulation phenotype in leaves and seeds (Lilay et al. 2021). The results obtained with the Col-0 background lines, which have a regular activity of native bZIP19 and bZIP23 transcription factors, supports that bZIP19-ZSM deletion represents a gain-of-function dominant mutation. This nature of the ZSM mutations might be advantageous in translational approaches from the Arabidopsis model to crops, targeting F-bZIPs activity in order to impact Zn nutritional value (Assunção 2022).
Conclusion
In this study, plant developmental traits and ionome of soil-grown Arabidopsis lines constitutively expressing bZIP19 with the ZSM deleted were analysed to investigate the effect of deregulation of the Zn deficiency response. Results indicated that this deregulation does not appear to cause penalties in plant growth, development, and reproduction. Ionomic analysis showed that deletion of the bZIP19-ZSM substantially increases Zn accumulation in leaves (ca. 2.5-fold) and seeds (ca. 1.5-fold) compared with the wild-type, and showed that this increased accumulation is largely Zn-specific. In seeds, Zn is distributed throughout the embryo in bZIP19-ZSM deletion line, comparably to wild-type. Exposure to low-level Cd contamination did not lead to enhanced Cd accumulation in the shoot nor did it affect the Zn accumulation pattern in the bZIP19-ZSM deletion lines, which is important given that uptake of the toxic metal Cd is a concern in crop Zn biofortification efforts. Future studies under controlled Zn supply at different concentrations, enabling deficiency and excess conditions, will provide additional insight and allow evaluating the potential and practicality of variations in the bZIP19-ZSM as a means to achieve plant Zn biofortification. Considering the evolutionary conservation of the plant F-bZIP-regulated Zn deficiency response (Castro et al. 2017), our results support the proposal that modulation of F-bZIP transcription factors activity is a promising avenue for Zn biofortification in crops.
References
Andreini C, Banci L, Bertini I, Rosato A (2006) Zinc through the three domains of life. J Proteome Res 5:3173–3178. https://doi.org/10.1021/pr0603699
Assunção AGL (2022) The F-bZIP-regulated Zn deficiency response in land plants. Planta 256:108. https://doi.org/10.1007/s00425-022-04019-6
Assunção AGL, Herrero E, Lin Y-F et al (2010) Arabidopsis thaliana transcription factors bZIP19 and bZIP23 regulate the adaptation to zinc deficiency. Proc Natl Acad Sci U S A 107:10296–10301. https://doi.org/10.1073/pnas.1004788107
Assunção AGL, Persson DP, Husted S et al (2013) Model of how plants sense zinc deficiency. Metallomics 5:1110–1116. https://doi.org/10.1039/c3mt00070b
Assunção AGL, Cakmak I, Clemens S et al (2022) Micronutrient homeostasis in plants for more sustainable agriculture and healthier human nutrition. J Exp Bot 73:1789–1799. https://doi.org/10.1093/jxb/erac014
Bentsink L, Yuan K, Koornneef M, Vreugdenhil D (2003) The genetics of phytate and phosphate accumulation in seeds and leaves of Arabidopsis thaliana, using natural variation. Theor Appl Genet 106:1234–1243. https://doi.org/10.1007/s00122-002-1177-9
Boyes DC, Zayed AM, Ascenzi R et al (2001) Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell 13:1499–1510. https://doi.org/10.1105/tpc.13.7.1499
Cailliatte R, Schikora A, Briat JF et al (2010) High-Affinity Manganese Uptake by the Metal Transporter NRAMP1 Is Essential for Arabidopsis Growth in Low Manganese Conditions. Plant Cell 22:904–917. https://doi.org/10.1105/tpc.109.073023
Castro PH, Lilay GH, Munoz-Merida A et al (2017) Phylogenetic analysis of F-bZIP transcription factors indicates conservation of the zinc deficiency response across land plants. Sci Rep 7:3806. https://doi.org/10.1038/s41598-017-03903-6
Castro PH, Lilay GH, Assuncao AGL (2018) Regulation of micronutrient homeostasis and deficiency response in plants. In: Hossain MA, Kamiya T, Burrit DJ, Phan Tran L-S, Fujiwara T (ed) Plant micronutrient use efficiency: molecular and genomic perspectives in crop plants. Academic Press Books, Elsevier, pp 1–15
Chang J-D, Huang S, Wiseno I et al (2023) Dissecting the promotional effect of zinc on cadmium translocation from roots to shoots in rice. J Exp Bot. https://doi.org/10.1093/jxb/erad330
Chao D-Y, Silva A, Baxter I et al (2012) Genome-wide association studies identify heavy metal ATPase3 as the primary determinant of natural variation in leaf cadmium in Arabidopsis thaliana. PLoS Genet 8:e1002923. https://doi.org/10.1371/journal.pgen.1002923
Chen H, Zhang W, Yang X et al (2018) Effective methods to reduce cadmium accumulation in rice grain. Chemosphere 207:699–707. https://doi.org/10.1016/j.chemosphere.2018.05.143
Clemens S (2001) Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212:475–486
Clemens S, Aarts MGM, Thomine S, Verbruggen N (2013a) Plant science: the key to preventing slow cadmium poisoning. Trends Plant Sci 18:92–99. https://doi.org/10.1016/j.tplants.2012.08.003
Clemens S, Deinlein U, Ahmadi H et al. (2013b) Nicotianamine is a major player in plant Zn homeostasis. Biometals 26:623–632. https://doi.org/10.1007/s10534-013-9643-1
Eroglu S, Giehl RFH, Meier B et al (2017) Metal Tolerance Protein 8 Mediates Manganese Homeostasis and Iron Reallocation during Seed Development and Germination. Plant Physiol 174:1633–1647. https://doi.org/10.1104/pp.16.01646
Guerinot ML (2000) The ZIP family of metal transporters. Biochim Biophys Acta 1465:190–198
Hussain D, Haydon MJ, Wang Y et al (2004) P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis. Plant Cell 16:1327–1339. https://doi.org/10.1105/tpc.020487
Kim SA, Punshon T, Lanzirotti A et al (2006) Localization of Iron in Arabidopsis Seed Requires the Vacuolar Membrane Transporter VIT1. Science(80-) 314:1295–1298. https://doi.org/10.1126/science.1132563
Lee S, Lee J, Ricachenevsky FK et al (2021) Redundant roles of four ZIP family members in zinc homeostasis and seed development in Arabidopsis thaliana. Plant J. https://doi.org/10.1111/tpj.15506
Lilay CPH, Campilho A, Assunção AGL (2019) The Arabidopsis bZIP19 and bZIP23 Activity Requires Zinc Deficiency – Insight on Regulation From Complementation Lines. Front Plant Sci 9:1955. https://doi.org/10.3389/fpls.2018.01955
Lilay PDP, Castro PH et al (2021) Arabidopsis bZIP19 and bZIP23 act as zinc sensors to control plant zinc status. Nat Plants 7:137–143. https://doi.org/10.1038/s41477-021-00856-7
Lin Y-F, Liang H-M, Yang S-Y et al (2009) Arabidopsis IRT3 is a zinc-regulated and plasma membrane localized zinc/iron transporter. New Phytol 182:392–404. https://doi.org/10.1111/j.1469-8137.2009.02766.x
Lindsay WL, Norvell WA (1978) Development of a DTPA Soil Test for Zinc, Iron, Manganese, and Copper. Soil Sci Soc Am J 42:421–428. https://doi.org/10.2136/sssaj1978.03615995004200030009x
McLaughlin MJ, Smolders E, Zhao FJ et al (2021) Managing cadmium in agricultural systems. Adv Agron 166:1–129. https://doi.org/10.1016/bs.agron.2020.10.004
Ministry of the Environment, Finland (2007) Government decree on the assessment of soil contamination and remediation needs (214/2007)
Morel M, Crouzet J, Gravot A et al (2009) AtHMA3, a P1B-ATPase allowing Cd/Zn/Co/Pb vacuolar storage in Arabidopsis. Plant Physiol 149:894–904. https://doi.org/10.1104/pp.108.130294
Olsen LI, Hansen TH, Larue C et al (2016) Mother-plant-mediated pumping of zinc into the developing seed. Nat Plants 2:16036. https://doi.org/10.1038/nplants.2016.36
Otegui MS, Capp R, Staehelin LA (2002) Developing Seeds of Arabidopsis Store Different Minerals in Two Types of Vacuoles and in the Endoplasmic Reticulum. Plant Cell 14:1311–1327. https://doi.org/10.1105/tpc.010486
Pita-Barbosa A, Ricachenevsky FK, Wilson M et al (2019) Transcriptional plasticity buffers genetic variation in zinc homeostasis. Sci Rep 9:19482. https://doi.org/10.1038/s41598-019-55736-0
Prasad AS (2009) Impact of the Discovery of Human Zinc Deficiency on Health. J Am Coll Nutr 28:257–265. https://doi.org/10.1080/07315724.2009.10719780
Robberse JG, Denneman CAJ (1993) Do target values help to protect the soil? In: Arendt F et al (ed) Contaminated soil’93, vol 2. Springer Netherlands, pp 373–382. https://doi.org/10.1007/978-94-011-2018-0_54
Sinclair SA, Krämer U (2012) The zinc homeostasis network of land plants. Biochim Biophys Acta 1823:1553–1567. https://doi.org/10.1016/j.bbamcr.2012.05.016
Thomine S, Wang R, Ward JM et al (2000) Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc Natl Acad Sci U S A 97:4991–4996
Vert G, Grotz N, Dédaldéchamp F et al (2002) IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14:1223–1233. https://doi.org/10.1105/tpc.001388
Welch RM, Graham RD (2004) Breeding for micronutrients in staple food crops from a human nutrition perspective. J Exp Bot 55:353–364. https://doi.org/10.1093/jxb/erh064
Wessells KR, Brown KH (2012) Estimating the Global Prevalence of Zinc Deficiency: Results Based on Zinc Availability in National Food Supplies and the Prevalence of Stunting. PLoS ONE 7:e50568. https://doi.org/10.1371/journal.pone.0050568
Funding
Open access funding provided by Copenhagen University. This work was supported by the Independent Research Fund Denmark (DFF-FTP, Grant No. 9041-00182B) and by the EU-ERASMUS-plus tranship grant. Elemental analysis was performed at CHEMI Center, University of Copenhagen, and at the ICP-MS Analysis Facility, School of Biosciences, University of Nottingham. LA-ICP-MS analysis was performed at the CHEMI Center. We thank Louise Jørgensen, Lena Byrgesen and Jannie Jessen for performing the analysis of total C, N and pH from soil.
Author information
Authors and Affiliations
Contributions
S. H. and A.G.L.A. designed the experimental work, analysed and interpreted data and wrote the manuscript. S. H. performed the experiments, prepared the samples for LA-ICP-MS and performed bright field microscopy. D.P performed the LA-ICP-MS. All authors revised and approved the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Responsible Editor: Fangjie Zhao.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huizinga, S., Persson, D.P. & Assunção, A.G.L. Constitutive expression of bZIP19 with the Zn sensor motif deleted in Arabidopsis leads to Zn-specific accumulation and no visible developmental penalty. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06729-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06729-0