Abstract
Purpose
The effects of trees on soil nematode communities are related to nutrient cycles in forest ecosystems. We conducted greenhouse pot experiments to determine the effects of a single tree species for each of coniferous and broad-leaved tree on soil nematodes.
Methods
Soils were collected from a coniferous plantation and broad-leaved forests. Seedlings of a coniferous tree (Cryptomeria japonica) and a broad-leaved tree (Quercus serrata) were planted in soils derived from each species. After 11 months, seedling biomass, soil properties, and ectomycorrhizal fungal colonization of Q. serrata were measured. Soil nematodes were morphologically identified to the genus/family level and differentiated by community and trophic composition.
Results
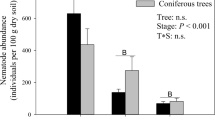
C. japonica root biomass was significantly higher than that of Q. serrata regardless of the soil and nematode community structures were significantly different between the species. The fungal: bacterial ratio and density of fungivorous nematodes were significantly higher in broad-leaved soils. Herbivorous nematodes increased significantly in C. japonica seedlings grown in broad-leaved soils. Structural equation modeling indicated that soil origin and tree species directly regulated nematode trophic compositions.
Conclusion
Our findings suggest that tree species modify soil micro-food webs by affecting microbial abundance and nematode trophic composition. Specifically, C. japonica, with a larger root biomass, increased the number of herbivorous nematodes, whereas Q. serrata, with ectomycorrhizal fungal symbiosis, increased the number of fungivorous nematodes. Thus, tree species are tightly involved in shaping nematode communities in forest ecosystems through root traits and mycorrhizal types.



Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Baermann G (1917) Eine einfache Methode zur Auffindung von Ancylostomum (Nematoden) larven in Erdproben. Geneeskd Tijdschr Ned Indie 57:131–137
Berg G, Grube M, Schloter M, Smalla K (2014) Unraveling the plant microbiome: looking back and future perspectives. Front Microbiol 5:1–7. https://doi.org/10.3389/fmicb.2014.00148
Bilgrami AL, Brey C, Gaugler R (2008) First field release of a predatory nematode, Mononchoides gaugleri (Nematoda: Diplogastrida), to control plant-parasitic nematodes. Nematology 10:143–146. https://doi.org/10.1163/156854108783360177
Bonkowski M, Villenave C, Griffiths B (2009) Rhizosphere fauna: the functional and structural diversity of intimate interactions of soil fauna with plant roots. Plant Soil 321:213–233. https://doi.org/10.1007/s11104-009-0013-2
Bongers T (1988) The nematodes of the Netherlands. The nematodes of the Netherlands. KNNV Publishing, Zeist
Bongers T, Bongers M (1998) Functional diversity of nematodes. Appl Soil Ecol 10:239–251. https://doi.org/10.1016/S0929-1393(98)00123-1
Cabos RYM, Wang KH, Sipes BS, Heller WP, Matsumoto TK (2013) Detection of plant parasitic nematode DNA in the gut of predatory and omnivorous nematodes. Nematropica 43:44e48
Cáceres MD, Legendre P (2009) Associations between species and groups of sites: indices and statistical inference. Ecology 90:3566–3574. https://doi.org/10.1890/08-1823.1
Cesarz S, Ruess L, Jacob M, Jacob A, Schaefer M, Scheu S (2013) Tree species diversity versus tree species identity: driving forces in structuring forest food webs as indicated by soil nematodes. Soil Biol Biochem 62:36–45. https://doi.org/10.1016/j.soilbio.2013.02.020
Cavard X, Macdonald SE, Bergeron Y, Chen HY (2011) Importance of mixedwoods for biodiversity conservation: evidence for understory plants, songbirds, soil fauna, and ectomycorrhizae in northern forests. Environ Rev 19:142–161. https://doi.org/10.1139/a11-004
Chemidlin Prévost-Bouré N, Christen R, Dequiedt S, Mougel C, Leliévre M, Jolivet C, Shahbazkia HR, Guillou L, Arrouays D, Ranjard L (2011) Validation and application of a PCR primer set to quantify fungal communities in the soil environment by real-time quantitative PCR. PLoS One 6:e24166. https://doi.org/10.1371/journal.pone.0024166
De Deyn GB, Raaijmakers CE, Van Ruijven J, Berendse F, van der Putten WH (2004) Plant species identity and diversity effects on different trophic levels of nematodes in the soil food web. Oikos 106:576–586. https://doi.org/10.1111/j.0030-1299.2004.13265.x
De Deyn GB, Cornelissen JHC, Bardgett RD (2008) Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol Lett 11:516–531. https://doi.org/10.1111/j.1461-0248.2008.01164.x
De Schrijver A, Vesterdal L, Hansen K, De Frenne P, Augusto L, Achat DL, Staelens J, Baeten L, De Keersmaeker L, De Neve S, Verheyen K (2012) Four decades of post-agricultural forest development have caused major redistributions of soil phosphorus fractions. Oecologia 169:221–234. https://doi.org/10.1007/s00442-011-2185-8
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366. https://doi.org/10.1890/0012-9615(1997)067[0345:SAAIST]2.0.CO;2
Eagar AC, Mushinski RM, Horning AL, Smemo KA, Phillips RP, Blackwood CB (2022) Arbuscular mycorrhizal tree communities have greater soil fungal diversity and relative abundances of saprotrophs and pathogens than ectomycorrhizal tree communities. Appl Environ Microbiol 88:e01782–e01721. https://doi.org/10.1128/AEM.01782-21
Eissenstat DM, Yanai RD (1997) The ecology of root lifespan. Adv Ecol Res 27:1–60
Ferris H (2010) Form and function: metabolic footprints of nematodes in the soil food web. Eur J Soil Biol 46:97–104. https://doi.org/10.1016/j.ejsobi.2010.01.003
Fierer N, Jackson JA, Vilgalys R, Jackson RB (2005) Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl Environ Microbiol 71:4117–4120. https://doi.org/10.1128/AEM.71.7.4117-4120.2005
Fierer N (2017) Embracing the unknown: disentangling the complexities of the soil microbiome. Nat Rev Microbiol 15:579–590. https://doi.org/10.1038/nrmicro.2017.87
Forest Agency of Japan (2022) Annual report on trends in forests and forestry 2022. Tokyo (in Japanese)
Forest Soil Division (1976) Classification of forest soils in Japan. Bull Gov For Exp Sta 280:1–28 (in Japanese with English summary)
Gebremikael MT, Steel H, Buchan D, Bert W, De Neve S (2016) Nematodes enhance plant growth and nutrient uptake under C and N-rich conditions. Sci Rep 6:32862. https://doi.org/10.1038/srep32862
Gilarte P, Pendall E, Carrillo Y, Nielsen UN (2021) Plant functional identity has predictable effects on nematode communities across successional stages. Soil Biol Biochem 162:108406. https://doi.org/10.1016/j.soilbio.2021.108406
Grace JB, Allain LK, Sankaran M, Knops J, Weiher E, Ritchie M, Anderson TM, Meche G, Andelman SJ, Smith MD, Jutila H, Seabloom E, Willig MR (2007) Does species diversity limit productivity in natural grassland communities? Ecol Lett 10:680–689. https://doi.org/10.1111/j.1461-0248.2007.01058.x
Heděnec P, Nilsson LO, Zheng H, Gundersen P, Schmidt IK, Rousk J, Vesterdal L (2020) Mycorrhizal association of common European tree species shapes biomass and metabolic activity of bacterial and fungal communities in soil. Soil Biol Biochem 149:107933. https://doi.org/10.1016/j.soilbio.2020.107933
Ingham RE, Trofymow JA, Ingham ER, Coleman DC (1985) Interactions of bacteria, fungi, and their nematode grazers: effects on nutrient cycling and plant growth. Ecol Monogr 55:119–140. https://doi.org/10.2307/1942528
Keith AM, Brooker RW, Osler GHR, Chapman SJ, Burslem DFRP, van der Wal R (2009) Strong impacts of belowground tree inputs on soil nematode trophic composition. Soil Biol Biochem 41:1060–1065. https://doi.org/10.1016/j.soilbio.2009.02.009
Kitagami Y, Matsuda Y (2020) Temperature changes affect multi-trophic interactions among pines, mycorrhizal fungi, and soil nematodes in a microcosm experiment. Pedobiologia 78:150595. https://doi.org/10.1016/j.pedobi.2019.150595
Kitagami Y, Tanikawa T, Matsuda Y (2020) Effects of microhabitats and soil conditions on structuring patterns of nematode communities in Japanese cedar (Cryptomeria japonica) plantation forests under temperate climate conditions. Soil Biol Biochem 151:108044. https://doi.org/10.1016/j.soilbio.2020.108044
Kitagami Y, Matsuda Y (2022) Effect of ectomycorrhizal fungal species on population growth and food preference of a fungivorous nematode. Mycorrhiza 32:95–104. https://doi.org/10.1007/s00572-021-01063-0
Klironomos JN, Hart MM (2001) Animal nitrogen swap for plant carbon. Nature 410:651–652. https://doi.org/10.1038/35070643
Kondratow F, Chauvin C, Villenave C, Andrieu E, Brin A (2019) Nematode communities after the reintroduction of silver fir in beech-dominated forests. Eur J For Res 138:957–965. https://doi.org/10.1007/s10342-019-01216-z
Kudrin AA, Zuev AG, Taskaeva AA, Konakova TN, Kolesnikova AA, Gruzdev IV, Gabov DN, Yakovleva EV, Tiunov AV (2021) Spruce girdling decreases abundance of fungivorous soil nematodes in a boreal forest. Soil Biol Biochem 155:108184. https://doi.org/10.1016/j.soilbio.2021.108184
Lefcheck JS (2016) piecewiseSEM: piecewise structural equation modelling in R for ecology, evolution, and systematics. Meth Evol Ecol 7:573–579. https://doi.org/10.1111/2041-210X.12512
Li Y, Du X, Su X, Han X, Liang W, Wang Z, Bruelheide H, Bezemer TM, Li Q (2023) Local-scale soil nematode diversity in a subtropical forest depends on the phylogenetic and functional diversity of neighbor trees. Plant Soil 486:441–454. https://doi.org/10.1007/s11104-023-05882-2
Liu T, Hu F, Li H (2019) Spatial ecology of soil nematodes: perspectives from global to micro scales. Soil Biol Biochem 137:107565. https://doi.org/10.1016/j.soilbio.2019.107565
Liu J, Wang X, Kou Y, Zhao W, Liu Q (2023) Differences in the effects of broadleaf and coniferous trees on soil nematode communities and soil fertility across successional stages. Plant Soil 485:197–212. https://doi.org/10.1007/s11104-022-05677-x
Maboreke HR, Graf M, Grams TEE, Herrmann S, Scheu S, Ruess L (2017) Multitrophic interactions in the rhizosphere of a temperate forest tree affect plant carbon flow into the belowground food web. Soil Biol Biochem 115:526–536. https://doi.org/10.1016/j.soilbio.2017.09.002
Mamilov AS, Dilly OM (2002) Soil microbial eco-physiology as affected by short-term variations in environmental conditions. Soil Biol Biochem 34:1283–1290. https://doi.org/10.1016/S0038-0717(02)00071-8
Mamiya Y (1967) Descriptive notes on three species of Trichodorus (Dorylaimide : Trichodoridae) from Forest nurseries in Japan. Appl Entomol Zool 2:61–68. https://doi.org/10.1303/aez.2.61
Matsuda Y, Takano Y, Shimada H, Yamanaka T, Ito SI (2013) Distribution of ectomycorrhizal fungi in a Chamaecyparis obtusa stand at different distances from a mature Quercus serrata tree. Mycoscience 54:260–264. https://doi.org/10.1016/j.myc.2012.09.019
Matsuda Y, Kita K, Kitagami Y, Tanikawa T (2021) Colonization status and community structure of arbuscular mycorrhizal fungi in the coniferous tree, Cryptomeria japonica, with special reference to root orders. Plant Soil 468:423–438. https://doi.org/10.1007/s11104-021-05147-w
Neher DA, Wu J, Barbercheck ME, Anas O (2005) Ecosystem type affects interpretation of soil nematode community measures. Appl Soil Ecol 30:47–64. https://doi.org/10.1016/j.apsoil.2005.01.002
Oksanen J, Simpson G, Blanchet F, Kindt R, Legendre P, Minchin P, O'Hara R, Solymos P, Stevens M, Szoecs E, Wagner H, Barbour M, Bedward M, Bolker B, Borcard D, Carvalho G, Chirico M, De Caceres M, Durand S, Evangelista H, FitzJohn R, Friendly M, Furneaux B, Hannigan G, Hill M, Lahti L, McGlinn D, Ouellette M, Ribeiro Cunha E, Smith T, Stier A, Ter Braak C, Weedon J (2022) Package “vegan.” Community Ecol. Package version 2.6–4. https://CRAN.R-project.org/package=vegan. Accessed 6 Dec 2023
Ostonen I, Püttsepp Ü, Biel C, Alberton O, Bakker M, Lõhmus K, Majdi H, Metcalfe D, Olsthoorn A, Pronk A (2007) Specific root length as an indicator of environmental change. Plant Biosyst 141:426–442. https://doi.org/10.1080/11263500701626069
Pierret A, Gonkhamdee S, Jourdan C, Maeght JL (2013) IJ_Rhizo: an open-source software to measure scanned images of root samples. Plant Soil 373:531–539. https://doi.org/10.1007/s11104-013-1795-9
Qian H, Hu B, Cao D, Chen W, Xu X, Lu Y (2007) Bio-safety assessment of validamycin formulation on bacterial and fungal biomass in soil monitored by real-time PCR. Bull Environ Contam Toxicol 78:239–244. https://doi.org/10.1007/s00128-007-9148-0
R Development Core Team (2023) R: a language and environment for statistical computing. R foundation for statistical computing, Austria. https://www.R-project.org/. Accessed 6 Dec 2023
Richter A, Ewald M, Hemmerling C, Schöning I, Bauhus J, Schall P, Ruess L (2023) Effects of management intensity, soil properties and region on the nematode communities in temperate forests in Germany. For Ecol Manag 529:120675. https://doi.org/10.1016/j.foreco.2022.120675
Ruess L, Zapata EJG, Dighton J (2000) Food preferences of a fungal-feeding Aphelenchoides species. Nematology 2:223–230. https://doi.org/10.1163/156854100508962
Salamon JA, Wolters V (2009) Nematoda response to forest conversion. Eur J Soil Biol 45:184–191. https://doi.org/10.1016/j.ejsobi.2008.09.014
Saucet SB, van Ghelder C, Abad P, Duval H, Esmenjaud D (2016) Resistance to root-knot nematodes Meloidogyne spp. in woody plants. New Phytol 211:41–56. https://doi.org/10.1111/nph.13933
Sawada K, Inagaki Y, Sugihara S, Funakawa S, Ritz K, Toyota K (2021) Impacts of conversion from natural forest to cedar plantation on the structure and diversity of root-associated and soil microbial communities. Appl Soil Ecol 167:104027. https://doi.org/10.1016/j.apsoil.2021.104027
Scherber C, Eisenhauer N, Weisser WW, Schmid B, Voigt W, Fischer M, Schulze ED, Roscher C, Weigelt A, Allan E, Beler H, Bonkowski M, Buchmann N, Buscot F, Clement LW, Ebeling A, Engels C, Halle S, Kertscher I et al (2010) Bottom-up effects of plant diversity on multitrophic interactions in a biodiversity experiment. Nature 468:553–556. https://doi.org/10.1038/nature09492
Schneider G, Chicken E, Becvarik R, Schneider MG (2021) Functions and datasets to accompany Hollander, Wolfe, and Chicken—nonparametric statistical methods. R Package Version 1:16
Shipley B (2009) Confirmatory path analysis in a generalized multilevel context. Ecology 90:363–368. https://doi.org/10.1890/08-1034.1
Soil Survey Staff (2014) Keys to soil taxonomy, 11th edn. USDA-Natural Resources Conservation Service, Washington, DC
Stefanowicz AM, Rożek K, Stanek M, Rola K, Zubek S (2021) Moderate effects of tree species identity on soil microbial communities and soil chemical properties in a common garden experiment. For Ecol Manag 482:118799. https://doi.org/10.1016/j.foreco.2020.118799
Tanikawa T, Maie N, Fujii S, Sun L, Hirano Y, Mizoguchi T, Matsuda Y (2023) Contrasting patterns of nitrogen release from fine roots and leaves driven by microbial communities during decomposition. Sci Total Environ 855:158809. https://doi.org/10.1016/j.scitotenv.2022.158809
Tedersoo L, Bahram M, Zobel M (2020) How mycorrhizal associations drive plant population and community biology. Science 367:1–9. https://doi.org/10.1126/science.aba1223
Thakur MP, Geisen S (2019) Trophic regulations of the soil microbiome. Trends Microbiol 27:771–780. https://doi.org/10.1016/j.tim.2019.04.008
van Bezooijen J (2006) Methods and techniques for nematology. Wageningen University, Wageningen, pp 30–33
van den Hoogen J, Geisen S, Routh D, Ferris H, Traunspurger W, Wardle DA, de Goede RGM, Adams BJ, Ahmad W, Andriuzzi WS, Bardgett RD, Bonkowski M, Campos-Herrera R, Cares JE, Caruso T, de Brito CL, Chen X, Costa SR, Creamer R et al (2019) Soil nematode abundance and functional group composition at a global scale. Nature 572:194–198. https://doi.org/10.1038/s41586-019-1418-6
Wada R, Tanikawa T, Doi R, Hirano Y (2019) Variation in the morphology of fine roots in Cryptomeria japonica determined by branch order-based classification. Plant Soil 444:139–151. https://doi.org/10.1007/s11104-019-04264-x
Wilschut RA, Geisen S, Martens H, Kostenko O, de Hollander M, ten Hooven FC, Weser C, Snoek LB, Bloem J, Caković D, Čelik T, Koorem K, Krigas N, Manrubia M, Ramirez KS, Tsiafouli MA, Vreš B, van der Putten WH (2019) Latitudinal variation in soil nematode communities under climate warming-related range-expanding and native plants. Glob Change Biol 25:2714–2726. https://doi.org/10.1111/gcb.14657
Wilschut RA, Geisen S (2021) Nematodes as drivers of plant performance in natural systems. Trends Plant Sci 26:237–247. https://doi.org/10.1016/j.tplants.2020.10.006
Wyss U (1997) Root parasitic nematodes: an overview. In: Fenoll C, Grundler FMW, Ohl SA (eds) Cellular and molecular aspects of plant-nematode interactions. Kluwer Academic Publishers, Dordrecht, pp 5–22
Yeates GW, Bongers T, De Goede RGM, Freckman DW, Georgieva SS (1993) Feeding habits in soil nematode families and genera—an outline for soil ecologists. J Nematol 25:315–331
Zhang X, Ferris H, Mitchell J, Liang W (2017) Ecosystem services of the soil food web after long-term application of agricultural management practices. Soil Biol Biochem 111:36–43. https://doi.org/10.1016/j.soilbio.2017.03.017
Zhang C, Wang J, Ren Z, Hu Z, Tian S, Fan W, Chen X, Griffiths BS, Hu F, Liu M (2020) Root traits mediate functional guilds of soil nematodes in an ex-arable field. Soil Biol Biochem 151:108038. https://doi.org/10.1016/j.soilbio.2020.108038
Zhang J, Hu Z, Zhang C, Tao Y, Chen X, Griffiths BS, Liu M (2022) Roots with larger specific root length and C: N ratio sustain more complex rhizosphere nematode community. Plant Soil 477:693–706. https://doi.org/10.1007/s11104-022-05465-7
Acknowledgments
We thank Mr. G. Yamanaka (The Mie Prefectural Forestry Research Center) for permission to access study sites. We also thank members of the laboratory of Forest Mycology at Mie University for their support with field sampling. We would like to thank Editage (www.editage.com) for English language editing. This study was supported in part by the Grants-in-Aid for Scientific Research (B) 18H02237, 21H02232 to YM and JSPS Research Fellow 18 J13285, Grant-in-Aid for Young Scientists 21 K14876 to YK from the Japan Society for the Promotion of Science.
Funding
This study was supported in part by the Grants-in-Aid for Scientific Research (B) 18H02237, 21H02232 to YM and JSPS Research Fellow 18 J13285, Grant-in-Aid for Young Scientists 21 K14876 to YK from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by YK, KS and YM. The first draft of the manuscript was written by YK and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Additional information
Responsible Editor: Sven Marhan.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(XLSX 41 kb)
ESM 2
Fig. S1. A conceptual figure of pot experiment. (a) 4 soil and tree species treatment combinations were established; Cryptomeria japonica soil with C. japonica seedlings (CC); Quercus serrata soil with C. japonica seedlings (QC); C. japonica soil with Q. serrata seedlings (CQ); Q. serrata soil with Q. serrata seedlings (QQ). (b) C. japonica and Q. serrata seedlings in pots grown for 11 months. (c) Procedures of experimental set-up. Fig. S2. Hypothesized initial full model. Soil origins and tree species were indicated as a categorical explanatory variable, i.e. soil origins; C. japonica (1) and Q. serrata (2), tree species; C. japonica (1) and Q. serrata (2). Principal component analysis (PCA) was performed to reduce the dimensions of soil abiotic factors, i.e., soil pH and C/N. The first principal component of soil properties (Soil PC1) was negatively correlated with soil pH and C/N ratio (see Fig. S3). Bact, abundance of bacterivores; Fung, abundance of fungivores; Herb, abundance of herbivores; Pred&Omni, abundance of predators and omnivores. Fig. S3. Principal component analysis of soil abiotic factors (soil pH and C/N ratio) from 96 pots. Cryptomeria japonica soil with C. japonica seedlings (CC); Quercus serrata soil with C. japonica seedlings (QC); C. japonica soil with Q. serrata seedlings (CQ); Q. serrata soil with Q. serrata seedlings (QQ). Fig. S4. Non-metric multidimensional scaling scatterplot of Chao dissimilarity based on the abundance of nematode taxa derived from four treatments and no seedling pots as control. Stress value = 0.227. Treatment codes show Cryptomeria japonica soil with C. japonica seedlings (CC); Quercus serrata soil with C. japonica seedlings (QC); C. japonica soil with Q. serrata seedlings (CQ); Q. serrata soil with Q. serrata seedlings (QQ). The “origin” showed the dataset at the time of sampling. The “control” showed the dataset of no tree treatments. Nematode community structures were clustered significantly into each treatment (PERMANOVA, P < 0.001, R2 = 0.73). Fig. S5. Number of total nematodes and four trophic groups per 100-g dry soil at four different treatments and no seedling pots as control. Columns are means (n = 24) with standard errors excepting for the number of control pots (n = 9). Treatment codes show Cryptomeria japonica soil with C. japonica seedlings (CC); Quercus serrata soil with C. japonica seedlings (QC); C. japonica soil with Q. serrata seedlings (CQ); Q. serrata soil with Q. serrata seedlings (QQ). C and Q indicate no tree treatments, i.e., only C. japonica and Q. serrata soils, respectively. (PPTX 452 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kitagami, Y., Suzuki, K. & Matsuda, Y. Effects of tree species identity and soil origin on soil nematode communities and trophic composition in coniferous and broad-leaved forests. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06599-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06599-6