Abstract
Background and aims
Anthracnose, caused by Colletotrichum graminicola, can greatly reduce yield in maize and alternatives for its management are needed. The hypothesis that maize plants with higher foliar silicon (Si) concentration will increase their resistance against anthracnose was investigated.
Methods
Plants were grown in substrate non-supplied or supplied with Si (-Si and + Si plants, respectively) and used to evaluate: (i) the photosynthetic apparatus (leaf gas exchange and chlorophyll (Chl) a fluorescence parameters and photosynthetic pigments concentration), (ii) the activities of defense and antioxidant enzymes and (iii) concentrations of malondialdehyde (MDA) and hydrogen peroxide (H2O2).
Results
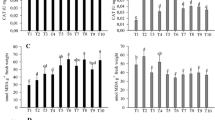
The + Si plants showed reduced anthracnose symptoms, lower severity and less production of MDA and H2O2 due to higher foliar Si concentration compared to -Si plants. The + Si infected plants showed less impairment in photosynthesis (increased values for rate of net CO2 assimilation, stomatal conductance to water vapor, transpiration rate, variable-to-maximum chlorophyll a fluorescence ratio, photochemical yield and yield for dissipation by down-regulation) and showed higher concentrations of chlorophyll a + b and carotenoids compared to -Si infected plants. Activities of defense (chitinase, β-1,3-glucanase, phenylalanine ammonia-lyase, polyphenoloxidase and lipoxygenase) and antioxidative (ascorbate peroxidase, catalase, superoxide dismutase and peroxidase) enzymes were higher for infected + Si plants compared to infected -Si plants.
Conclusion
Maize supplied with Si had their resistance boosted due to a more operative defense response, a robust antioxidative metabolism and a better photosynthetic performance. Using Si to reduce anthracnose epidemic rate and yield losses should be encouraged to promote a more sustainable agriculture.











Similar content being viewed by others
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Aucique-Pérez CE, Rodrigues FA, Moreira WR, DaMatta FM (2014) Leaf gas exchange and chlorophyll a fluorescence in wheat plants supplied with silicon and infected with Pyricularia oryzae. Phytopathology 104:143–149
Aucique-Pérez CE, Resende RS, Neto LBC, Dornelas F, DaMatta FM, Rodrigues FA (2019) Picolinic acid spray stimulates the antioxidative metabolism and minimizes impairments on photosynthesis on wheat leaves infected by Pyricularia oryzae. Physiol Plant 167:628–644
Bergstrom GC, Nicholson RL (1999) The biology of corn anthracnose: knowledge to exploit for improved management. Plant Dis 83:596–608
Bermúdez-Cardona MB, Wordell Filho JA, Rodrigues FA (2015) Leaf gas exchange and chlorophyll a fluorescence in maize leaves infected with Stenocarpella macrospora. Phytopathology 105:26–34
Costa RV, Cota LV, Silva DD, Parreira DF, Casela CR, Landau EC, Figueiredo JEF (2014) Races of Colletotrichum graminicola pathogenic to maize in Brazil. Crop Prot 56:44–49
Cota V, Costa RV, Silva DD, Casela CR, Parreira DF (2012) Quantification of yield losses due to anthracnose stalk rot on corn in Brazilian conditions. J Phytopathol 160:680–684
Debona D, Rodrigues FA, Rios JA, Nascimento KJT (2012) Biochemical changes in the leaves of wheat plants infected by Pyricularia oryzae. Phytopathology 102:1121–1129
Debona D, Rodrigues FA, Rios JA, Martins SCV, Pereira LF, DaMatta FM (2014) Limitations to photosynthesis in leaves of wheat plants infected by Pyricularia oryzae. Phytopathology 104:34–39
Debona D, Rodrigues FA, Datnoff LE (2017) Silicon’s role in abiotic and biotic plant stresses. Annu Rev Phytopathol 55:85–107
Domiciano GP, Cacique IS, Freitas CC, Filippi MCC, DaMatta FM, Vale FXR, Rodrigues FA (2015) Alterations in gas exchange and oxidative metabolism in rice leaves infected by Pyricularia oryzae are attenuated by silicon. Phytopathology 105:738–747
Erenstein O, Jaleta M, Sonder K, Mottaleb K, Prasanna BM (2022) Global maize production, consumption and trade: trends and R&D implications. Food Security 14:1295–1319
Hansen TH, Bang TC, Laursen KH, Pedas P, Husted S, Schjoerring JK (2013) Multielement plant tissue analysis using ICP spectrometry. Methods Mol Biol 953:121–141
Hawerroth C, Araujo L, Bermúdez-Cardona MB, Silveira PR, Wordell Filho JA, Rodrigues FA (2019) Silicon-mediated maize resistance to macrospora leaf spot. Tropical Plant Pathology 44:192–196
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. In: Circular california agricultural experiment station, 2nd edn. California, USA
Kaur S, Samota MH, Choudhary M, Choudhary M, Pandey AK, Sharma A, Thakur J (2022) How do plants defend themselves against pathogens - Biochemical mechanisms and genetic interventions. Physiol Mol Biol Plants 28:485–504
Korndörfer GH, Pereira HS, Nolla A (2004) Análise de Silício: Solo, Planta e Fertilizante. Uberlândia: Grupo de Pesquisa em Silício, Instituto de Ciências Agrárias, Universidade Federal de Uberlândia, 34 p., Boletim Técnico 2
Ma JF, Yamaji N (2015) A cooperative system of silicon transport in plants. Trends Plant Sci 20:435–442
Mims CW, Vaillancourt LJ (2002) Ultrastructural characterization of infection and colonization of maize leaves by Colletotrichum graminicola, and by a C. graminicola pathogenicity mutant. Phytopathology 92:803–812
Moore KJ, Dixon PM (2015) Analysis of combined experiments revisited. Agron J 107:763–771
O’Connell RJ, Thon MR, Hacquard S, Amyotte SG, Kleemann J, Torres MF, Vaillancourt LJ (2012) Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nat Genet 44:1060–1065
Parreira DF, Zambolim L, Costa RV, Silva DD, Marcondes MM, Lanza FE, Neves WS, Figueiredo JEF, Souza AGC, Cota LV (2016) A method for Colletotrichum graminicola inoculation in maize stalks. Revista Brasileira De Milho e Sorgo 15:53–64
Picanço BBM, Silva BN, Rodrigues FA (2022) Potentiation of soybean resistance against Phakopsora pachyrhizi infection using phosphite combined with free amino acids. Plant Pathol 71:1496–1510
Resende RS, Rodrigues FA, Cavatte PC, Martins SCV, Moreira WR, Chaves ARM, DaMatta FM (2012) Leaf gas exchange and oxidative stress in sorghum plants supplied with silicon and infected by Colletotrichum sublineolum. Phytopathology 102:892–898
Rios JA, Rodrigues FA, Debona D, Silva LC (2014) Photosynthetic gas exchange in leaves of wheat plants supplied with silicon and infected with Pyricularia oryzae. Acta Physiol Plant 36:371–379
Rodrigues FA, Dallagnol LJ, Duarte HSS, Datnoff LE (2015) Silicon control of foliar diseases in monocots and dicots. In: Rodrigues FA, Datnoff LE (eds) Silicon and Plant Diseases. Springer, pp 67–108
Rodrigues FA, Resende RS, Dallagnol LJ, Datnoff LE (2015) Silicon potentiates host defense mechanisms against infection by plant pathogens. In: Rodrigues FA, Datnoff LE (eds) Silicon and Plant Diseases. Springer, pp 109–138
Rodrigues FCT, Aucique-Pérez CE, Fontes BA, Ribeiro DM, Rodrigues FA (2020) Involvement of ethylene in wheat resistance to infection by Pyricularia oryzae. Physiol Mol Plant Pathol 112:101526
Rodrigues FA, Bispo W, Aucique-Pérez CE (2014) The effect of silicon on plant photosynthesis during pathogens infection. In: Photosynthesis: functional genomics, physiological processes and environmental issues, 1st edn. Nova Science Publishers, New York, pp 211–220
Silveira PR, Nascimento KJT, Andrade CCL, Bispo WMS, Oliveira JR, Rodrigues FA (2015) Physiological changes in tomato leaves arising from Xanthomonas gardneri infection. Physiol Mol Plant Pathol 92:130–138
Silveira PR, Milagres PO, Corrêa EF, Aucique-Pérez CE, Wordell Filho JA, Rodrigues FA (2019) Changes in leaf gas exchange, chlorophyll a fluorescence, and antioxidants in maize leaves infected by Exserohilum turcicum. Biol Plant 63:643–653
Silveira PR, Aucique-Pérez CE, Cruz MFA, Rodrigues FA (2021) Biochemical and physiological changes in maize plants supplied with silicon and infected by Exserohilum turcicum. J Phytopathol 169:393–408
Tatagiba SD, Rodrigues FA, Filippi MCC, Silva GB, Silva LC (2014) Physiological responses of rice plants supplied with silicon to Monographella albescens infection. J Phytopathol 162:596–606
Tatagiba SD, DaMatta FM, Rodrigues FA (2015) Leaf gas exchange and chlorophyll a fluorescence imaging of rice leaves infected with Monographella albescens. Phytopathology 105:180–188
Tatagiba SD, DaMatta FM, Rodrigues FA (2016) Silicon partially preserves the photosynthetic performance of rice plants infected by Monographella albescens. Annals of Applied Biology 168:111–121
Acknowledgements
Prof. Rodrigues thanks the National Council for Technological and Scientific Development (CNPq) for his fellowship. Grants from FAPEMIG and CNPq supported this study. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mochko, A.C.R., Silva, B.N., Oliveira, L.M. et al. Silicon-mediated resistance in maize against infection by Colletotrichum graminicola. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06586-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06586-x