Abstract
Aims
Chemical defence in plant tissue is an important physiological process in plants, mainly driven by secondary metabolites. However, how the chemical defence substances regulate soil biological processes after tissue death is still unclear.
Methods
Litter bags filled with cotton strips and wood sticks were placed on the forest floor, combined soil zymography and high-throughput sequencing methods, we studied the effect of chemical defence substances of Cinnamomum camphora on the decomposition of the standard litter incubated in C. camphora forest and Quercus variabilis forest. In laboratory trials, we tested how microorganisms responded to these chemical defence substances using selective media.
Results
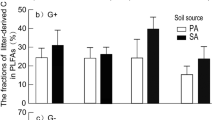
The addition of C. camphora chemical defence substances increased the decomposition rate of standard litter in situ environment by more than 70%; While in the away ecosystem, Q. variabilis forest, reduced the decomposition rate of standard litter by approximately 50%. The soil P content and activity of acid phosphatase changed significantly. Adding C. camphora components to the C. camphora forest significantly enhanced the abundance of detritivores, the opposite result is observed in Q. variabilis forest. Evidence suggests that C. camphora components regulated soil microbial communities.
Conclusions
Our study suggests a legacy effect associated with plant chemical defence substances, which regulates the soil nutrient cycle such as litter decomposition and plays a positive or negative role in soil carbon dynamics depending on the presence of co-evolved or naïve decomposers. The study of this universal ecological legacy effect provides an important theoretical reference for soil utilization and planting of plantations.
Graphical abstract






Similar content being viewed by others
References
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79(3):439–449
Ågren GI (2008) Stoichiometry and nutrition of plant growth in natural communities. Annu Rev Ecol Evol Syst 39(1):153–170
Aguilera N, Becerra J, Villaseñor-Parada C, Lorenzo P, González L, Hernández V (2015) Effects and identification of chemical compounds released from the invasive Acacia dealbata link. Chem Ecol 31(6):479–493
Anderegg WRL, Schwalm C, Biondi F, Camarero JJ, Koch G, Litvak M, Ogle K, Shaw JD, Shevliakova E, Williams AP, Wolf A, Ziaco E, Pacala S (2015) Pervasive drought legacies in forest ecosystems and their implications for carbon cycle models. Science 349(6247):528
Asensio D, Yuste JC, Mattana S, Ribas À, Llusià J, Peñuelas J (2012) Litter VOCs induce changes in soil microbial biomass C and N and largely increase soil CO2 efflux. Plant Soil 360(1):163–174
Austin AT, Ballaré CL (2010) Dual role of lignin in plant litter decomposition in terrestrial ecosystems. Proc Natl Acad Sci 107(10):4618–4622
Barbehenn RV, Peter Constabel C (2011) Tannins in plant-herbivore interactions. Phytochemistry 72(13):1551–1565
Berg B, McClaugherty C (2020) Introduction. In: Pages 1–12 Plant litter: decomposition, humus formation, carbon sequestration. Springer International Publishing, Cham
Bezemer TM, van Dam NM (2005) Linking aboveground and belowground interactions via induced plant defenses. Trends Ecol Evol 20(11):617–624
Bonanomi G, Zotti M, Idbella M, Mazzoleni S, Abd-ElGawad AM (2021) Microbiota modulation of allelopathy depends on litter chemistry: mitigation or exacerbation? Sci Total Environ 776:145942
Bradford MA, Berg B, Maynard DS, Wieder WR, Wood SA (2016) Understanding the dominant controls on litter decomposition. J Ecol 104(1):229–238
Cao T, Fang Y, Chen Y, Kong X, Yang J, Alharbi H, Kuzyakov Y, Tian X (2022a) Synergy of saprotrophs with mycorrhiza for litter decomposition and hotspot formation depends on nutrient availability in the rhizosphere. Geoderma 410:115662
Cao T, Kong X, He W, Chen Y, Fang Y, Li Q, Chen Q, Luo Y, Tian X (2022b) Spatiotemporal characteristics of enzymatic hotspots in subtropical forests: in situ evidence from 2D zymography images. Catena 216:106365
Chollet S, Maillard M, Schorghuber J, Grayston SJ, Martin JL (2021) Deer slow down litter decomposition by reducing litter quality in a temperate forest. Ecology 102(2):e03235
Chomel M, Guittonny-Larchevêque M, Fernandez C, Gallet C, DesRochers A, Paré D, Jackson BG, Baldy V (2016) Plant secondary metabolites: a key driver of litter decomposition and soil nutrient cycling. J Ecol 104(6):1527–1541
Cleveland CC, Liptzin D (2007) C:N:P stoichiometry in soil: is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 85(3):235–252
Coley PD, Bryant JP, Chapin FS (1985) Resource availability and plant antiherbivore defense. Science 230(4728):895
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Pérez-Harguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, Van Bodegom P, Brovkin V, Chatain A et al (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11(10):1065–1071
Cuddington K (2011) Legacy effects: the persistent impact of ecological interactions. Biol Theory 6(3):203–210
Descombes P, Pitteloud C, Glauser G, Defossez E, Kergunteuil A, Allard PM, Rasmann S, Pellissier L (2020) Novel trophic interactions under climate change promote alpine plant coexistence. Science 370(6523):1469–1473
Dicke M, Baldwin IT (2010) The evolutionary context for herbivore-induced plant volatiles: beyond the 'cry for help'. Trends Plant Sci 15(3):167–175
Dong S, Brooks D, Jones MD, Grayston SJ (2007) A method for linking in situ activities of hydrolytic enzymes to associated organisms in forest soils. Soil Biol Biochem 39(9):2414–2419
Feeny P (1970) Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51(4):565–581
Fine PVA, Mesones I, Coley PD (2004) Herbivores promote habitat specialization by trees in amazonian forests. Science 305(5684):663
Guo S, Geng Z, Zhang W, Liang J, Wang C, Deng Z, Du S (2016) The chemical composition of essential oils from Cinnamomum camphora and their insecticidal activity against the stored product pests. Int J Mol Sci 17(11):1836
Hamman ST, Hawkes CV (2013) Biogeochemical and microbial legacies of non-native grasses can affect restoration success. Restor Ecol 21(1):58–66
Hättenschwiler S, Vitousek PM (2000) The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol Evol 15(6):238–243
Hawlena D, Strickland MS, Bradford MA, Schmitz OJ (2012) Fear of predation slows plant-litter decomposition. Science 336(6087):1434–1438
Hu W, Gao H, Jiang X, Yang H (2012) Analysis on constituents and contents in leaf essential oil from three chemical types of Cinnamum camphora. J Central South Univ For Technol 32(11):186–194
Hu W, Dai C, Zhou S (2019) Research on major constituents, bacteriostatic effect and antibacterial mechanism of fractions from Cinnamomum longepaniculatum leaves essential oils and its major monomer components. Anhui Agric Sci Bull 25(15):14–19
Huang W, Hu T, Chen H, Wang Q, Hu H, Tu L, Jing L (2013) Impact of decomposing Cinnamomum septentrionale leaf litter on the growth of Eucalyptus grandis saplings. Plant Physiol Biochem 70:411–417
Idbella M, De Filippis F, Zotti M, Sequino G, Abd-ElGawad AM, Fechtali T, Mazzoleni S, Bonanomi G (2022) Specific microbiome signatures under the canopy of Mediterranean shrubs. Appl Soil Ecol 173:104407
Johnston ASA, Sibly RM (2020) Multiple environmental controls explain global patterns in soil animal communities. Oecologia 192(4):1047–1056
Kos M, Jing J, Keesmaat I, Declerck SAJ, Wagenaar R, Bezemer TM (2017) After-life effects: living and dead invertebrates differentially affect plants and their associated above- and belowground multitrophic communities. Oikos 126(6):888–899
Kraus TEC, Dahlgren RA, Zasoski RJ (2003) Tannins in nutrient dynamics of forest ecosystems - a review. Plant Soil 256(1):41–66
Leclercq-Dransart J, Demuynck S, Bidar G, Douay F, Grumiaux F, Louvel B, Pernin C, Leprêtre A (2019) Does adding fly ash to metal-contaminated soils play a role in soil functionality regarding metal availability, litter quality, microbial activity and the community structure of Diptera larvae? Appl Soil Ecol 138:99–111
Li S, Wang J, Chen C, Li H, Hao D (2022) Tolerance, biochemistry and related gene expression in Pagiophloeus tsushimanus (Coleoptera: Curculionidae) exposed to chemical stress from headspace host-plant volatiles. Agric For Entomol 24(2):189–203
Lin H, He Z, Hao J, Tian K, Jia X, Kong X, Akbar S, Bei Z, Tian X (2017) Effect of N addition on home-field advantage of litter decomposition in subtropical forests. For Ecol Manag 398:216–225
Lin H, Zhao Y, Muyidong N, Tian K, He Z, Kong X, Sun S, Tian X (2018) Secondary compounds of Pinus massoniana alter decomposers’ effects on Quercus variabilis litter decomposition. Ecol Evol 8(18):9439–9450
Liu C, Mishra AK, Tan R, Tang C, Yang H, Shen Y (2006) Repellent and insecticidal activities of essential oils from Artemisia princeps and Cinnamomum camphora and their effect on seed germination of wheat and broad bean. Bioresour Technol 97(15):1969–1973
Luo Y, Wang L, Cao T, Chen J, Lv M, Wei S, Lu S, Tian X (2023) Microplastics are transferred by soil fauna and regulate soil function as material carriers. Sci Total Environ 857:159690
Ma J, Quan Z, Sun Y, Du J, Liu B (2020) Excess sulfur and Fe elements drive changes in soil and vegetation at abandoned coal gangues. Guizhou China Scientific Reports 10(1):10456
Macfadyen A (1953) Notes on methods for the extraction of small soil arthropods. J Anim Ecol 22(1):65–77
McBride SG, Osburn ED, Barrett JE, Strickland MS (2019) Volatile methanol and acetone additions increase labile soil carbon and inhibit nitrification. Biogeochemistry 145(1):127–140
McBride SG, Choudoir M, Fierer N, Strickland MS (2020) Volatile organic compounds from leaf litter decomposition alter soil microbial communities and carbon dynamics. Ecology 101(10):e03130
Moles AT, Bonser SP, Poore AGB, Wallis IR, Foley WJ (2011) Assessing the evidence for latitudinal gradients in plant defence and herbivory. Funct Ecol 25(2):380–388
Moorhead DL, Sinsabaugh RL, Hill BH, Weintraub MN (2016) Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol Biochem 93:1–7
Mori, AS, Cornelissen JHC, Fujii S, Okada K-I, Isbell F (2020) A meta-analysis on decomposition quantifies afterlife effects of plant diversity as a global change driver. Nat Commun 11(1):4547
Nishida S, Naiki A, Nishida T (2005) Morphological variation in leaf domatia enables coexistence of antagonistic mites in Cinnamomum camphora. Can J Bot 83(1):93–101
Poudel DK, Rokaya A, Ojha PK, Timsina S, Satyal R, Dosoky NS, Satyal P, Setzer WN (2021) The chemical profiling of essential oils from different tissues of Cinnamomum camphora L. and their antimicrobial activities. Molecules 26(17):5132
Ramirez KS, Lauber CL, Fierer N (2010) Microbial consumption and production of volatile organic compounds at the soil-litter interface. Biogeochemistry 99(1):97–107
Randriamanana TR, Nybakken L, Lavola A, Aphalo PJ, Nissinen K, Julkunen-Tiitto R (2014) Sex-related differences in growth and carbon allocation to defence in Populus tremula as explained by current plant defence theories. Tree Physiol 34(5):471–487
Sanchez G (2013) PLS path modeling with R. Trowchez Editions, Berkeley
Shi L, Wang H, Fu X, Kou L, Meng W, Dai X (2020) Soil enzyme activities and their stoichiometry of typical plantations in mid-subtropical China. Chin J Appl Ecol 31(6):1980–1988
Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, Crenshaw C, Contosta AR, Cusack D, Frey S, Gallo ME, Gartner TB, Hobbie SE, Holland K, Keeler BL, Powers JS, Stursova M, Takacs-Vesbach C, Waldrop MP, Wallenstein MD et al (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11(11):1252–1264
Sinsabaugh RL, Hill BH, Shah JJF (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462(7274):795–798
Soler R, Erb M, Kaplan I (2013) Long distance root–shoot signalling in plant–insect community interactions. Trends Plant Sci 18(3):149–156
Spohn M, Kuzyakov Y (2014) Spatial and temporal dynamics of hotspots of enzyme activity in soil as affected by living and dead roots—a soil zymography analysis. Plant Soil 379(1):67–77
Tian K, Kong X, Yuan L, Lin H, He Z, Yao B, Ji Y, Yang J, Sun S, Tian X (2019) Priming effect of litter mineralization: the role of root exudate depends on its interactions with litter quality and soil condition. Plant Soil 440(1–2):457–471
Tiegs SD, Costello DM, Isken MW, Woodward G, McIntyre PB, Gessner MO, Chauvet E, Griffiths NA, Flecker AS, Acuna V, Albarino R, Allen DC, Alonso C, Andino P, Arango C, Aroviita J, Barbosa MVM, Barmuta LA, Baxter CV et al (2019) Global patterns and drivers of ecosystem functioning in rivers and riparian zones. Sci Adv 5(1):eaav0486
van der Plas F, Manning P, Allan E, Scherer-Lorenzen M, Verheyen K, Wirth C, Zavala MA, Hector A, Ampoorter E, Baeten L, Barbaro L, Bauhus J, Benavides R, Benneter A, Berthold F, Bonal D, Bouriaud O, Bruelheide H, Bussotti F et al (2016) Jack-of-all-trades effects drive biodiversity–ecosystem multifunctionality relationships in European forests. Nat Commun 7(1):11109
Wagg C, Bender SF, Widmer F, van der Heijden Marcel GA (2014) Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc Natl Acad Sci 111(14):5266–5270
Wang X, Liu J, Gao W, Deng Y, Ni Y, Xiao Y, Kang F, Wang Q, Lei J, Jiang Z (2016) Defense pattern of Chinese cork oak across latitudinal gradients: influences of ontogeny, herbivory, climate and soil nutrients. Sci Rep 6:27269
Witt B (1997) Using soil Fauna to improve soil health. Restoration and Reclamation Review 2(8):1–5
Wu K, Lin Y, Chai X, Duan X, Zhao X, Chun C (2019) Mechanisms of vapor-phase antibacterial action of essential oil from Cinnamomum camphora var. linaloofera Fujita against Escherichia coli. Food Sci Nutr 7(8):2546–2555
Yang J, Guo H, Jiang N, Tang R, Wang C (2021) Identification of a gustatory receptor tuned to sinigrin in the cabbage butterfly Pieris rapae. PLoS Genet 17(7):e1009527
Yuan Z (2021) Characteristics of carbon Pool and nutrients under two typical forests in Nanjing Zijin Mountain. MA thesis. Nanjing Forestry University. MA thesis
Zhang D, Hui D, Luo Y, Zhou G (2008) Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. J Plant Ecol 1(2):85–93
Acknowledgements
Sincere thanks to the National Natural Science Foundation of China (31870598), Jiangsu Forestry Science and technology innovation and promotion project (LYKJ[2021]16), Strategic Priority Research Program of the Chinese Academy of Sciences (A) (XDA19050400), Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX22_0128) and the program B for Outstanding PhD candidate of Nanjing University (202201B049 and 202201B051) for financially supporting the study. We thank Run Liu and Yunru Chen for their guidance in the experiment, Hongjun Liu and Yigui Zhang for their field help, and Xiao-Fen Li for data collection. Special thanks are extended to Dr. Björn Berg for his critical reading and comments on revising the manuscript.
Author information
Authors and Affiliations
Contributions
Y.L. conceived of the study. Y.L. and X.T. designed the study. Y.L., L.W., F.L., S.L. and Z.Z. conducted the field experiments. Y.L., W.H., S.L. and F.L. conducted the lab experiments. Y.L. and L.W. analysed the results. Tinging C. provided guidance for the experiment. Tong C. drew the figures. Y.L. wrote the manuscript. Tinging C., L.W., W.H. and X.T. edited the manuscript. All authors approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflicting interests.
Additional information
Responsible Editor: Alfonso Escudero.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 2941 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, Y., Wang, L., Cao, T. et al. Legacy effect of plant chemical defence substances on litter decomposition. Plant Soil 487, 93–108 (2023). https://doi.org/10.1007/s11104-023-05954-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-05954-3