Abstract
Purpose
Root exudation of organic acids (OAs) facilitates plant P uptake from soil, playing a key role in rhizosphere nutrient availability. However, OA exudation responses to CO2 concentrations and water availability remain largely untested.
Methods
We examined the effects of CO2 and water on OA exudates in three Australian woodland species: Eucalyptus tereticornis, Hakea sericea and Microlaena stipoides. Seedlings were grown in a glasshouse in low P soil, exposed to CO2 (400 ppm [aCO2] or 540 ppm [eCO2]) and water treatments (100% water holding capacity [high-watered] or 25–50% water holding capacity [low-watered]). After six weeks, we collected OAs from rhizosphere soil (OArhizo) and trap solutions in which washed roots were immersed (OAexuded).
Results
For E. tereticornis, the treatments changed OArhizo composition, driven by increased malic acid in plants exposed to eCO2 and increased oxalic acid in low-watered plants. For H. sericea, low-watered plants had higher OAexuded per plant (+ 116%) and lower OArhizo per unit root mass (–77%) associated with larger root mass but fewer cluster roots. For M. stipoides, eCO2 increased OAexuded per plant (+ 107%) and per unit root mass (+ 160%), while low-watered plants had higher citric and lower malic acids for OArhizo and OAexuded: changes in OA amounts and composition driven by malic acid were positively associated with soil P availability under eCO2.
Conclusion
We conclude that eCO2 and altered water availability shifted OAs in root exudates, modifying plant–soil interactions and the associated carbon and nutrient economy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding how terrestrial ecosystems respond to global changes requires an accurate prediction of plant responses to increases in atmospheric CO2 concentrations and changes in water availability. While elevated CO2 concentrations (eCO2) generally enhance photosynthetic rates and increase plant growth, multiple lines of evidence suggest that the plant response to eCO2 depends on soil nutrient status: the positive effect of eCO2 on plant growth weakens under nutrient-limited conditions but strengthens with increased nutrient availability (Reich et al. 2006). Thus, soil nutrient economy and plant nutrient uptake strategy likely drive the magnitude of plant responses to eCO2. However, little is known about how future atmospheric CO2 concentrations will affect these factors (Walker et al. 2021).
Plants exhibit various strategies to acquire nutrients when soil availability is low, for example, by increasing their relative belowground carbon (C) investment (higher root:shoot ratios) or adjusting their root morphology (Hill et al. 2010; Ostonen et al. 2011). Soil nutrient acquisition can also be enhanced via plant investment of photosynthates to support mycorrhizae (Eissenstat et al. 2015; Helmisaari et al. 2009). Other strategies to increase nutrient availability include secretion of readily available organic C (i.e. sugars) from roots that acts as substrates for rhizosphere microbes, enhancing their decomposition of organic matter—so-called ‘priming’ (Kuzyakov 2010; van Hees et al. 2005). Finally, plants can exude low-molecular-weight organic anions (organic acids; OAs) from roots to mobilise P. Phosphate bound to cations (e.g. Mn2+, Al3+, Ca2+) or adsorbed onto soil mineral surfaces is inaccessible to plants. However, OAs compete with phosphate, preventing it from forming complexes with cations and thus liberating P (Bais et al. 2006). Interestingly, P mobilisation efficiency varies among different OAs (Mendes et al. 2020). Thus, changes in the amount and composition of root-exuded OAs likely impact soil P cycling and associated plant growth.
Soils in Australia are typically impoverished, particularly in P availability (Walker and Syers 1976). Therefore, native plant species in Australia are well-adapted to low P conditions, with a range of P acquisition strategies such as high rates of OA exudation and mycorrhizal symbiosis (Lambers et al. 2012; Ryan et al. 2012). Indeed, some species in the Cyperaceae and Proteaceae families are particularly well-adapted to P-limited conditions and form cluster roots that express especially high rates of OA exudation (Lambers et al. 2012).
Elevated CO2 likely increases plant nutrient demands due to enhanced growth and/or altered tissue stoichiometry (Bassirirad et al. 1996; Dijkstra et al. 2012; Johnson et al. 2004). Plants typically invest excess C belowground to tackle increased nutrient demands, enhancing microbial decomposition of organic matter and ectomycorrhizal nutrient transport (Terrer et al. 2016). Hasegawa et al. (2016) and Ochoa-Hueso et al. (2017) reported eCO2 increased soil P availability in a free-air CO2 enrichment (FACE) experiment established in a P-limited Eucalyptus woodland in Australia (EucFACE). These studies put forward the hypothesis that increased photosynthetic rates under eCO2 conditions increase root secretion of OAs into the soil, liberating soil P in line with the expectations from Jin et al. (2015), Lambers et al. (2008) and Ryan et al. (2012).
In combination with eCO2 and global warming, substantial changes in precipitation patterns linked to climate change cause extreme rainfall events in many parts of the world (Sun et al. 2021). In particular, the southeastern parts of Australia have experienced prolonged periods of drought and intensified rainfall accompanied by flooding on an unprecedented scale in recent years (Australian Bureau of Meteorology: http://www.bom.gov.au). These extreme events are projected to be exacerbated in the future (Grose et al. 2020), and thus there is an urgent need to investigate the role of climate in modulating plant nutrient uptake strategies. However, few studies have scrutinised the responses of OAs in root exudates to different levels of water availability; those that have studied such effects on OA exudation report drought stress increased OA amounts and shifted rhizosphere OA composition for leguminous species, despite lower photosynthetic rates (Sharma et al. 2021; Suriyagoda et al. 2010). Waterlogging stress, on the other, suppressed root exudation for rapeseed (Zhou et al. 1997) and soybean (Shimono et al. 2012). This contrasts with the hypothesised effects of eCO2 enhancing root exudation via increased photosynthetic rates (Phillips et al. 2011). Thus, CO2- and water-induced changes in OA exudates are likely regulated by different mechanisms but remain largely unexplored. Furthermore, little is known about the effects of CO2 and water availability on OA exudation.
Here, we implement two extreme scenarios for water availability: high-watered conditions (soil water held close to field capacity) and low-watered conditions (soil water availability maintained at a similar level to that of dry, warm seasons in local Eucalyptus woodlands in southeast Australia). We study how root exudation of OAs responds to eCO2 under these two water regimes using three plant species native to these woodlands: Eucalyptus tereticornis, Hakea sericea and Microlaena stipoides. Eucalyptus tereticornis thrives across eastern Australia (Drake et al. 2015) and is the dominant canopy species at the EucFACE field site. The genus Eucalyptus forms both arbuscularmycorrhizae and ectomycorrhizae (Boudarga et al. 1990; Chilvers et al. 1987). Hakea sericea is an understorey shrub species in the Proteaceae family. It is typically nonmycorrhizal but produces cluster roots and hence is considered well-adapted to low P conditions (Dinkelaker et al. 1995). Microlaena stipoides is a native, perennial C3 grass species, typically associated with arbuscular mycorrhizae (Hill et al. 2010). At the EucFACE site, M. stipoides is responsible for ~ 99% of the herbaceous understorey vegetation biomass (Collins et al. 2018; Hasegawa et al. 2018), contributing to 24% of the ecosystem gross primary productivity and thus playing a significant role in C cycling (Jiang et al. 2020).
We grew plants in a glasshouse in soil collected from the EucFACE field site, allowing us to relate results to earlier findings from the field study. We tested the hypotheses that: 1) eCO2 has positive effects on root exudation; 2) plants growing under low-watered conditions exude larger amounts of OAs than those under high water availability; 3) the magnitude of positive eCO2 effects on root exudation is smaller for low-watered than high-watered plants as eCO2 improves plant water use efficiency, leading to slower depletion of soil water under low-watered conditions (Robredo et al. 2007); and 4) CO2 and water treatments alter OA composition. Release of carboxylic acids from roots serves to maintain the charge balance in plant cells when cation and anion uptakes are unbalanced, and hence available N forms (nitrate or ammonium) can directly influence root exudation (Imas et al. 1997). Therefore, we further hypothesise 5) that CO2-/water-induced changes in OA amounts are concomitant with changes in soil nutrient availability, with larger OA amounts associated with higher nitrate and phosphate concentrations and lower ammonium concentrations.
Materials and methods
Experimental design, setup and plant growth
Three plant species were examined, each representing one stratum within the native Eucalyptus woodlands in southeastern Australia: E. tereticornis (canopy tree), H. sericea (understorey shrub) and M. stipoides (woodland floor graminoid). Seeds of E. tereticornis were purchased from a commercial supplier (Australian Tree Seed Centre, CSIRO National Research Collections Australia), while those of H. sericea and M. stipoides were collected locally in a native Eucalyptus woodland near Richmond, NSW (adjacent to the EucFACE field site). The seeds were sown and germinated on seed cell trays in growth chambers (30 °C for E. tereticornis, 25 °C for H. sericea and M. stipoides). Following germination, trays were transferred to a glasshouse bay. Once seedlings were well-established (ca. 2–3 weeks after germination), they were transplanted into cylindrical PVC pots (10 cm diameter × 33 cm height): 20 pots for E. tereticornis and 32 each for H. sericea and M. stipoides. The number of pots for E. tereticornis was fewer than for the other two species due to lower germination rates. One seedling was transplanted into each pot for E. tereticornis and H. sericea. Three seedlings for M. stipoides were planted into each pot to achieve a similar level of plant biomass per pot across all three species. Each cylindrical pot was filled with 3.6 kg soil collected adjacent to the EucFACE site (ca. 1.6 g cm–3 bulk density). The soil is a loamy sand Chromosol of the Clarendon Formation (Bannerman and Hazelton 1990; Barton et al. 2010) with a pH of 5.45, 677 mg kg–1 total N, 59 mg kg–1 total P and 1.8% organic matter content. A more detailed description of the soil is available elsewhere (Crous et al. 2015; Hasegawa et al. 2016). Soil was collected from the top 0–20 cm, air-dried for two days, sieved (4 mm) to remove plant material and rocks, and thoroughly homogenised before use. When transplanted into pots, seedlings were well-watered and allowed to settle for 2–3 days.
Pots were placed in one of four naturally lit glasshouse compartments located at Western Sydney University in Richmond, NSW, Australia, between March and April 2016. Detailed information on the management of glasshouse conditions is available elsewhere (Ghannoum et al. 2010). Briefly, the glasshouse temperature simulated the 30-year average daily temperature of the local region during the growing season (November–May), with average temperatures of 26 and 18 °C for day and night, respectively. The average relative humidity was 70%. Of the four compartments, two received ambient CO2 (400 ppm), and two received elevated CO2 (ambient + 140 ppm). CO2 concentrations in the eCO2 compartments were raised by injecting CO2 gas (Food grade, Air Liquid, Australia) from pressurised cylinders. The glasshouse temperature and CO2 concentrations were constantly monitored and regulated by a control system (Lambda T, ADC BioScientific Ltd., Hoddesdon, Herts, UK). The plants were exposed to two levels of water availability to examine the interactive effects between CO2 and water treatments. Half of the pots were highly watered (hereafter referred to as ‘high-watered’), with the soil water content maintained at 100% water holding capacity (WHC) (ca. 15% gravimetric water content [GWC] or 23% volumetric water content [VWC]). The other half of the pots were maintained at 50% WHC (ca. 7% GWC) for the first three weeks and then 25% WHC for the next three weeks until harvest (hereafter referred to as ‘low-watered’). The pots were weighed and watered three times a week with deionised water to meet the target soil water contents. Pots were grouped into blocks, each comprising high-watered and low-watered replicates, resulting in ten blocks for E. tereticornis and 16 blocks for H. sericea and M. stipoides. Half of the blocks received aCO2 and the other half eCO2. Blocks were randomly allocated to one of four glasshouse compartments, rotated within the compartments three times a week and between replicate compartments within the same CO2 treatment once a week to minimise potential heterogeneity of environmental conditions within and between replicate compartments.
Harvest and root exudate collection
After six weeks, the plants were harvested. All plants were watered one day before harvest so that the soil water contents were consistent at 50% WHC. Each pot was upended to remove the full plant and soil contents, and the soil was then gently shaken off the plant. The soil that remained on roots was defined as rhizosphere soil. Root exudates of OAs were collected following the approach in Kidd et al. (2018) using two sequential methods. First, roots were immersed in a known volume (ca. 50 ml) of 0.2 mM CaCl2 solution and gently shaken for 2–3 min to wash off rhizosphere soil. The collected OAs are referred to as rhizosphere OAs (OArhizo). Second, following removal of rhizosphere soil, roots were thoroughly washed with Milli-Q water, placed in a known volume (ca. 60 ml) of 0.2 mM CaCl2 solution, and incubated for 1 h at room temperature in a laboratory to collect exudates directly from roots, referred to as OAexuded. OAexuded represents the amount of OA exudates from plant roots over time, whereas OArhizo is the amount of OAs in rhizosphere soil as the net result of exudation and losses through microbial degradation and adsorption onto soil particles. Although carefully executed, the process of harvesting inevitably damaged some root cells, likely resulting in a minute metabolite leak into the collected OA solutions. The OArhizo and OAexuded solutions were filtered (0.2 µm) before adding a drop of concentrated phosphoric acid to each to minimise microbial decomposition. Solutions were immediately frozen in liquid nitrogen and stored at –20 °C until analysis. The harvested plants were placed in a drying oven at 80 °C for 2–3 days, with dry weight (DW) of above- and below-ground parts measured to obtain total biomass and root:shoot ratios (RSR). For H. sericea, the number of cluster roots was counted, and cluster root counts per unit root biomass were calculated.
Analysis of OAs in root exudates
Organic acids were analysed using Reverse Phase Liquid Chromatography as described in Cawthray (2003) for the following compounds: malic, acetic, citric, cis-aconitic, succinic, fumaric and trans-aconitic acids. Malonic and maleic acids were also analysed but not detected in the current study. Oxalic acid was measured following Uloth et al. (2015). Briefly, oxalic acid was separated at 23 °C on a Hypersil Hypercarb column using a mobile phase of 0.1% trifluoroacetic acid at 15 ml min–1. Detection was at 210 nm, with photodiode array detector acquisition from 195 to 400 nm. Total amounts of OArhizo and OAexuded equated to the sum of all detected OAs on a mass basis for each growing pot. Total OArhizo amount was expressed as µg plant–1, and total OAexuded amount was expressed as µg plant–1 h–1. Root-mass based amounts of OArhizo and OAexuded were expressed as µg g–1 root DW and µg g–1 root DW h–1, respectively. Total OA amounts were converted to C mass using molecular weights and the number of C atoms for each OA to estimate the amount of C secreted from roots as OAs.
Soil nutrients
Plant-accessible nutrients were evaluated using ion exchange resin membranes (IEMs) buried in the top 0–6 cm of each pot, after modifying the method outlined in Hovenden et al. (2008). Anion and cation IEMs were cut into 1 cm × 6 cm pieces. Anion membranes were placed on one side of a plastic tag and cation membranes on the other side, collectively representing an IEM-tag. Two IEM-tags were inserted into the soil in each pot at the start of the CO2 and water treatments. These tags were collected at harvest and washed with Milli-Q water to remove adhesive soil. Duplicate IEM-tags for each pot were placed in a falcon tube, mechanically shaken with 40 ml of 0.1 M hydrochloric acid for 1 h to extract nitrate (NO3–-N), ammonium (NH4+-N) and phosphate (PO43–-P). This solution was then diluted ten times to determine extracted N and P concentrations using a continuous flow auto-analyser (AQ2 Discrete Analyzer, SEAL Analytical, Mequon, WI, USA).
Statistical analysis
Statistical analyses were performed using R 4.1.2 (R Core Team 2021). We report results at the significance level of ⍺ = 0.05, and marginally significant results (P ≤ 0.1) as an important indicator of treatment effects. Each of the studied species was analysed separately. There were 20 pots for E. tereticornis and 32 for each of M. stipoides and H. sericea, resulting in ten and 16 blocks of paired pots (low- and high-watered pots), respectively. Hence, the water treatments had treatment replicates of ten for E. tereticornis and 16 for M. stipoides and H. sericea. There were two glasshouse compartments for each of the CO2 treatments, where blocks were regularly moved within and between the compartments to minimise variation in the measurements attributed to different compartments. Hence, each block (instead of the compartment) was treated as a replicate for CO2 treatment—five replicates for E. tereticornis and eight replicates for M. stipoides and H. sericea.
Linear mixed-effects models (LMMs) were performed using the ‘lme4’ package (Bates et al. 2015) to examine CO2, water and their interactive effects on OArhizo and OAexuded (total and root-mass based amount), with block as a random factor. LMMs were also used to evaluate treatment effects on plant/soil variables (total biomass, RSR and soil nitrate, ammonium and phosphate availability, and the number of cluster roots per unit root biomass for H. sericea). Response variables were transformed (loge, square root or Box-Cox transformation) as required to ensure homogeneity of variances and normality of errors prior to analysis (Crawley 2012; Fox and Weisberg 2011). P values of the fixed factors in the LMMs (i.e. CO2, water and CO2 × water) were approximated by F-test using Type II ANOVA tests with Kenward-Roger Degrees of Freedom, using the ‘car’ and ‘lmerTest’ packages (Fox and Weisberg 2011; Kenward and Roger 1997; Kuznetsova et al. 2017). The amounts of each OA in OArhizo and OAexuded were evaluated individually to examine the response of each OA to CO2 and water treatments. Since there were frequent zeros for some OAs (or not detectable) and the values were bound by zero, the distribution and error structure of the data were not normal. Therefore, we employed generalised linear mixed-effects models (GLMMs) using the Gamma distribution with log link, with block as a random factor. As the Gamma distribution can handle only non-zero positive values, a small value (i.e. the smallest value detected) was added to all OA values that had zeros prior to analysis. P values of the fixed factors in the GLMMs were obtained by Chi-squared tests.
Multiple regression analysis was performed to evaluate the associations between total OA amounts and plant/soil measurements for OArhizo and OAexuded. RSR and soil ammonium availability were loge-transformed prior to the analysis. Explanatory variables were Z-standardised so that values had mean = 0 and standard deviation = 1. Ninety-five percent confidence intervals (CIs) on the coefficients of the multiple regression model were estimated by parametric bootstrap with 999 simulations using the ‘lme4’ package. Multicollinearity between the explanatory variables was checked using variance inflation factors (≤ 5). Where block did not explain any of the variations in response variables and did not improve the models, this random factor was removed from the LMMs, GLMMs and multiple regression analysis, and (generalised) linear models were performed instead to avoid a singular fit (Bates et al. 2015).
Redundancy analysis (RDA) was employed to assess CO2 and water effects on the composition of OArhizo and OAexuded for each species using the ‘vegan’ package (Oksanen et al. 2020). Compositional OA data were Hellinger-transformed prior to analysis. The interactive term of the combined CO2 and water treatments did not improve adjusted R2 values for the tested models and hence was not included in the analysis (Legendre and Legendre 2012). RDA models were tested with 4,999 permutations. Additional permutation tests were performed when significant associations between the OA composition and CO2 and water treatments were indicated at ⍺ < 0.05 to analyse the marginal effects of each term. Correlations between the resulting RDA axes (RDA1 and RDA2) and plant/soil variables (total biomass, RSR, soil ammonium, nitrate and phosphate, and the number of cluster roots per unit root biomass for H. sericea) were also assessed. RSR was loge-transformed. Non-metric multidimensional scaling (NMDS) was used for OArhizo and OAexuded to visualise the pattern of OA composition across the studied species, with the relative abundance of each OA computed and Hellinger-transformed for each plant prior to analysis.
Results
Plant and soil measurements
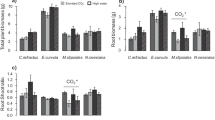
The effects of CO2 and water treatments on plant/soil measurements were species-specific. Total biomass generally increased in the eCO2 treatment, by 20% (P > 0.1), 29% (P < 0.05) and 33% (P < 0.1) relative to aCO2, for E. tereticornis, H. sericea and M. stipoides, respectively (Fig. 1a, Table S1). No eCO2 effects occurred for C investment in belowground biomass (i.e. RSR and the number of cluster roots per unit root biomass, Fig. 1b, c) or for soil nitrate concentrations in any studied species (Fig. 1d). The eCO2 treatment increased soil ammonium concentrations for E. tereticornis, relative to aCO2 (+ 139%, P < 0.001), while they did not change for H. sericea and decreased for M. stipoides (–49%, P < 0.001). The eCO2 effects on soil ammonium concentrations were particularly large for low-watered E. tereticornis and M. stipoides compared to high-watered pots, as indicated by a significant interaction for both species (Fig. 1e). Soil phosphate concentrations were unaffected by the eCO2 treatment for E. tereticornis or H. sericea, but significantly increased for M. stipoides (+ 79%, P < 0.01) relative to aCO2 (Fig. 1f, Table S1).
Plant and soil measurements for Eucalyptus tereticornis, Hakea sericea and Microlaena stipoides (mean ± 1 standard error, n = 5 for E. tereticornis and n = 8 for others): a) total biomass, b) root:shoot ratios (RSR), c) number of cluster roots per g root dry weight (DW), and concentrations of d) soil nitrate, e) ammonium and f) phosphate assessed using ion exchange resin membranes. Ambient (aCO2) and elevated (eCO2) CO2 treatments are depicted with open and filled boxes, respectively. High-watered (HW) and low-watered (LW) treatments are indicated by blue and red colours, respectively. The results of statistical analyses are shown for CO2 (C), water (W) and their interaction (CW) at the top of corresponding panels for each species, with significance codes: P < 0.1 (†), < 0.05 (*), < 0.01 (**) and < 0.001 (***)
Low-watered plants had significantly higher total biomass than high-watered E. tereticornis (+ 125%, P < 0.001), H. sericea (+ 31%, P < 0.01) and M. stipoides (+ 37%, P < 0.05) (Fig. 1a, Table S1). Low-watered E. tereticornis had significantly lower RSR (–18%, P < 0.05) than high-watered plants, while it was higher for H. sericea (+ 18%, P < 0.001) and did not change for M. stipoides (Fig. 1b). Low-watered H. sericea had significantly fewer cluster roots per unit root biomass (–44%, P < 0.001) than high-watered plants (Fig. 1c). The water treatments did not affect soil nitrate concentrations for E. tereticornis, whereas low-watered H. sericea and M. stipoides had significantly higher soil nitrate concentrations (+ 106%, P < 0.001 and + 111%, P < 0.001, respectively) than high-watered plants (Fig. 1d). In contrast, low-watered plants had significantly higher soil ammonium concentrations for E. tereticornis (+ 127%, P < 0.001) but lower concentrations for H. sericea (–4%, P < 0.05; removing one outlier in eCO2:High-watered: –30%, P < 0.01) and M. stipoides (–45%, P < 0.001) than high-watered plants (Fig. 1e). Low-watered plants had consistently lower soil phosphate concentrations than high-watered plants for all species: E. tereticornis (–52%, P < 0.001), H. sericea (–57%, P < 0.001) and M. stipoides (–43%, P < 0.001) (Fig. 1f, Table S1).
Quantitative responses of organic acids in root exudates
The OArhizo and OAexuded amounts varied among the three species but were within the same order of magnitude when expressed per unit root biomass (Fig. 2). On average, OArhizo amounts were 376 ± 73, 836 ± 368 and 479 ± 84 µg g–1 root DW for E. tereticornis, H. sericea and M. stipoides, respectively (mean ± 1 SE, Fig. S1). OAexuded amounts were 319 ± 72, 175 ± 24 and 249 ± 48 µg h–1 g–1 root DW for E. tereticornis, H. sericea and M. stipoides, respectively.
Total amounts of organic acids per plant a) in the rhizosphere soil (OArhizo) and b) exuded from roots (OAexuded) for Eucalyptus tereticornis, Hakea sericea and Microlaena stipoides (mean ± 1 standard error, n = 5 for E. tereticornis and n = 8 for others). Ambient (aCO2) and elevated (eCO2) CO2 treatments are depicted with open and filled boxes, respectively. High-watered (HW) and low-watered (LW) treatments are indicated by blue and red colours, respectively. Statistical significance is indicated for CO2 (C), water (W) and their interaction (CW) at the top of corresponding figures for each species: P < 0.1 (†), < 0.05 (*), < 0.01 (**) and < 0.001 (***)
Effect of CO2 and water treatments on exudation
For E. tereticornis and H. sericea, there was no evidence of eCO2 effects on the total amounts of OArhizo or OAexuded regardless of the units of expression (per plant or unit root biomass) (Figs. 2, S1, Table S2). For M. stipoides, eCO2 did not alter OArhizo amount but more than doubled OAexuded amount per plant (P < 0.05, Fig. 2) and per unit root biomass (P < 0.05, Fig. S1b, Table S2), increasing the amount of C secreted from roots from 2.6 to 5.6 ug C h–1 per plant (Fig. S2b).
Water treatments did not affect the total amount of OArhizo or OAexuded for E. tereticornis and M. stipoides, regardless of expressed units (per plant or unit root biomass) (Figs. 2, S1, Table S2). While this was also the case for total OArhizo amount per plant in H. sericea, (Fig. 2a), the low-watered plants had 77% less total OArhizo amount per unit root biomass than high-watered plants of this species (P < 0.05, Fig. S1a, Table S2), decreasing the amount of C in OArhizo from 470 to 104 µg C g–1 root DW (Fig. S2). Low-watered H. sericea plants also had greater OAexuded amounts per plant (+ 116%, P < 0.05, Fig. 2b) and per unit root biomass (+ 32%, P > 0.1, Fig. S1b) than high-watered plants, although the latter was not statistically significant (Table S2), increasing the amount of C in OAexuded from 4.7 to 9.4 µg C plant–1 h–1 (Fig. S2).
Association between exudates and plant/soil measurements
For E. tereticornis, total OArhizo amount had a significant positive association with total biomass, but total OAexuded did not correlate with any tested variable (Table S3). No associations were found between total OArhizo amount and any tested variable for H. sericia, but total OAexuded was positively correlated with total biomass and RSR (Table S3). OArhizo amount in M. stipoides positively correlated with total biomass and soil phosphate concentrations, and OAexuded amount was positively correlated with total biomass (Table S3).
Qualitative responses of organic acids in root exudates
OA compositions in OArhizo and OAexuded varied among the three species (Fig. 3). The exudates from M. stipoides mainly comprised malic acid (11–50% and 19–80% in OArhizo and OAexuded, respectively) and citric acid (7–54% and 0–45%) while H. sericea exudates were predominantly trans-aconitic acids (66–90% and 87–94%). Large amounts of oxalic and malic acids were also occasionally observed for H. sericea but only in a few pots, and this species was the only one to produce cis- and trans-aconitic acids. The exudates from E. tereticornis mainly comprised oxalic acid (27–96% and 51–84%) (Fig. 3a, b, Table S4).
Organic acid (OA) composition in root exudates in response to CO2 and water treatments. Relative abundance is shown in the upper panels for OAs a) in the rhizosphere soil (OArhizo) and b) exuded from roots (OAexuded). The lower panels depict ordination plots obtained from non-metric multidimensional scaling (NMDS) analysis for (c) OArhizo and (d) OAexuded, with centroids and associated standard errors for CO2 (ambient [aCO2] and elevated [eCO2] CO2) and water treatments (high-watered [HW] and low-watered [LW] conditions) for each species: Eucalyptus tereticornis, Hakea sericea and Microlaena stipoides. Organic acids are indicated in purple: acetic (Ace.), cis-aconitic (c-Aco.), citric (Cit.), fumaric (Fum.), malic (Mal.), oxalic (Oxa.), shikimic (Shi.) and trans-aconitic (t-Aco.) acids
Effect of CO2 and water treatments on OA composition
For E. tereticornis, the CO2 treatment significantly altered OArhizo composition (P < 0.01, Fig. 4a, Table S5), likely driven by increased malic acid at eCO2 relative to aCO2 (+ 322%, P < 0.05, Table S4), but did not affect OAexuded composition (P > 0.1, Fig. 4d). CO2 treatment did not affect OArhizo or OAexuded composition for H. sericea (Table S5, Fig. 4b, e). For M. stipoides, CO2 treatment significantly altered OArhizo composition (P < 0.05, Fig. 4c), despite no main CO2 effects on any detected OAs when analysed individually (Table S4). However, an interaction occurred between the CO2 and water treatments for citric acid in OArhizo for M. stipoides: eCO2 significantly increased the amount of citric acid relative to aCO2 under high-watered but not low-watered conditions (Table S4). A similar pattern occurred for malic acid in OArhizo, but this was not statistically significant. For M. stipoides, the CO2 treatment weakly influenced OAexuded composition (P < 0.1, Fig. 4f, Table S5), possibly due to changes in shikimic acid, as its exudation rate was sevenfold higher under eCO2 than aCO2 (Table S4).
Redundancy analysis (RDA) results of the effect of CO2 and water treatments on organic acid (OA) composition in the rhizosphere soil (OArhizo, upper panel) and exuded from roots (OAexuded, lower panel) for Eucalyptus tereticornis (a, d), Hakea sericea (b, e) and Microlaena stipoides (c, f). The results of permutation tests for the significance of constraints are shown for CO2 (C), water (W) and their interaction (CW) at the top of each panel, with significance codes. Proportion of variability in OA composition attributed to RDA axes is shown within parentheses. The correlation test results for the RDA axes and plant and environmental measurements are shown with arrows: root:shoot ratios (RS), total biomass (Biom), soil nitrate, ammonium and phosphate, and number of cluster roots per g root dry weight (CR; only for H. sericea), with significance codes. The significance codes are P < 0.1 (†), < 0.05 (*), < 0.01 (**) and < 0.001 (***). OAs are indicated in purple: acetic (Ace.), cis-aconitic (c-Aco.), citric (Cit.), fumaric (Fum.), malic (Mal.), oxalic (Oxa.), shikimic (Shi.) and trans-aconitic (t-Aco.) acids
The water treatment significantly altered OArhizo composition (P < 0.05, Fig. 4a, Table S5) in E. tereticornis, likely driven by increased oxalic acid under low-watered compared to high-watered conditions (+ 80%, P < 0.05, Table S4), but not OAexuded composition (P > 0.1, Fig. 4d, Table S5). Hakea sericea OArhizo and OAexuded composition were unaffected by the water treatment (Fig. 4b, e, Table S5). For M. stipoides, the water treatment significantly altered OArhizo composition (P < 0.05, Fig. 4c, Table S5), likely driven by increased citric acid (+ 100%, P < 0.05) and decreased malic (–45%, P < 0.01) and shikimic acids (–40%, P < 0.1) under low-watered compared to high-watered conditions (Table S4). Changes in oxalic acid may have also contributed to the shift in OArhizo composition as it was only observed in low-watered M. stipoides. For this species the water treatment also significantly altered OAexuded composition (P < 0.05, Fig. 4f, Table S5), likely due to increased citric acid under low-watered compared to high-watered plants (+ 215%, P < 0.05, Table S4). Furthermore, malic acid may have contributed to the compositional shift in OAexuded as indicated by its relatively larger negative value for the species score along the first RDA axis accounting for 11.0% of the total variability (Fig. 4f). The amount of malic acid in OAexuded for M. stipoides was so low that it was undetectable in some pots. While there was no evidence of CO2 or water treatment effects on malic acid, this OA was detected more often in high-watered plants at eCO2 (7 of 8 pots), with higher mean levels, than other conditions (2–3 of 8 pots) (Table S4).
Association between OA composition and plant/soil measurements
As mentioned above, the CO2 and water treatments significantly altered OArhizo composition in E. tereticornis, with the RDA axes correlated with soil phosphate concentrations (P < 0.05) and plant biomass (P < 0.1, Fig. 4a). Along the first RDA axis, accounting for 24% of the total variation in data, soil phosphate concentrations negatively correlated with oxalic acid concentrations in low-watered pots under aCO2 and positively correlated with malic acid in high-watered pots under eCO2 (also see Fig. 3a). For M. stipoides, the CO2 and water treatments strongly influenced OArhizo and OAexuded composition, with RDA axes strongly correlated with changes in total biomass and soil phosphate concentrations (Fig. 4c, f); under eCO2:High-watered conditions (with a relatively high proportion of malic acid) OA composition correlated with high soil phosphate concentrations, whereas under eCO2:Low-watered conditions (with a relatively larger proportion of citric acid) OA composition correlated with high total biomass (also see Fig. 3a, b).
Discussion
We performed a unique study demonstrating CO2 concentration and water availability caused quantitative and qualitative changes in OA root exudates. Plants grown under low-watered conditions outperformed those under high-watered conditions for all three species. The H. sericea and M. stipoides seeds used in our study were collected from the local native Eucalyptus woodland where soil rarely reaches its full WHC (Hasegawa et al. 2018). Thus, the provenances of H. sericea and M. stipoides used in this study may have been well-adapted to the local hot, dry climate with well-drained sandy soil (Pathare et al. 2017; Piñeiro et al. 2021). Meanwhile, insufficient water availability is generally known to inhibit the growth of a wide range of Eucalyptus species (Atwell et al. 2007; Ngugi et al. 2003). It is possible that high-watered plants in this study experienced anaerobic soil conditions. Associated negative effects of plant pathogens may also have played a role, although there were no visual signs of fungal infection on any of the studied plants.
Organic acid exudation of the studied species
Overall, the total amount of OAs in root exudates (on a per plant basis) was mainly driven by plant size: larger plants within the same species had higher exudation rates. Also, significant shifts in OA composition in response to CO2 and/or water treatments were accompanied by changes in plant biomass. Thus, CO2 and water availability likely indirectly influence OA amount and composition through altered plant size (Aulakh et al. 2001; Calvo et al. 2019).
Kidd et al. (2018) collected OAs in root exudates from 10 pasture legume species using the same technique as the present study, reporting OArhizo amounts from 12–191 µmol g–1 root DW which are generally larger amounts than herein (3.5–6.7 µmol g–1 root DW; data not shown). However, when expressed on a root length basis, instead of root mass basis, OA amounts in Kidd et al. (2018) ranged from < 1–14 nmol cm–1 root (except Cicer arietinum L. with 68 nmol cm–1 root), which are similar to our amounts (7–13 nmol cm–1 root, data not shown). Our study species had lower specific root lengths (or thicker roots) and hence smaller surface area per unit of root mass than those in Kidd et al. (2018). These differences in root architecture may explain differences between our native woodland species and the pasture legumes reported in Kidd et al. (2018). It should also be noted that the growth media differed between the two studies: Kidd et al. (2018) grew plants in washed river sand, which minimises the adsorption and microbial decomposition of OAs, likely resulting in greater amounts of rhizosphere OA than those in native soil (Ryan et al. 2012).
Roelofs et al. (2001) evaluated OA exudation from seven Australian Proteaceae (Banksia, Hakea and Dryandra) species grown in nutrient solutions, reporting OA exudation rates from cluster roots ranging from 1.0–2.5 nmol g−1 root fresh weight s–1. If root DW is assumed to be 10% of fresh weight, as assumed by Roelofs et al. (2001), these figures equate to 36–90 µmol g–1 root DW h–1, which is higher than the value for H. sericea in this study (6.7 ± 2.9 µmol g–1 root DW h–1, mean ± 1 standard error, data not shown). This was likely due to our collection of root exudates from the whole root system, including cluster and non-cluster roots, whereas Roelofs et al. (2001) only sampled from cluster roots. The OAexuded from H. sericea in our study was predominantly represented by trans-aconitic acid, while citric and malic acids were undetected. This compositional pattern is more similar to non-cluster than cluster roots (Roelofs et al. 2001), suggesting that cluster roots may have played a relatively small role in OAs of root exudates in the present study.
Organic acid composition for the two OA collection methods (OArhizo or OAexuded) were generally in agreement for the studied species (Fig. 3a, b), suggesting that the loss of each OA by microbial degradation or mineral adsorption in the rhizosphere was in proportion to what were produced by plants and hence the treatment effects were similar for those two measures. However, for M. stipoides acetic acid was observed in OArhizo but not in OAexuded: this likely reflected acetic acid being derived from rhizosphere microbes (Suriyagoda et al. 2016). On the other hand, fumaric acid from M. stipoides and E. tereticornis was less abundant in OArhizo than OAexuded, suggesting it was rapidly degraded by microbes or adsorbed onto soil particles.
Effect of CO2 treatment on exudation
For E. tereticornis, there was no evidence of a CO2 effect on OA amounts in root exudates. Given the lack of CO2 effect on total biomass or RSR, these results suggest that E. tereticornis did not alter its belowground C allocation to facilitate increased nutrient uptake in response to eCO2.
For H. sericea, there was no evidence of CO2 effects on OAexuded or OArhizo amount. There was also no evidence of a CO2 effect on cluster root production, which contrasts with Campbell and Sage (2002) who reported an increased number of cluster roots in white lupin (Lupinus albus) in response to eCO2 using a hydroponic technique. A plethora of hydroponic studies report increased cluster root production with reduced P supply (e.g. Neumann et al. 1999; Shane et al. 2003). As argued above, the OA composition of H. sericea in this study was similar to non-cluster roots rather than cluster roots. Combined with a significant increase in biomass of under eCO2, it appears that H. sericea had adequate access to P without the need to invest C into further cluster root production or root exudation.
Increased OAexuded amounts in M. stipoides under eCO2 were likely due to increased photosynthetic rates as observed in the field study where there was a 28% increase in net CO2 assimilation rates (Pathare et al. 2017). Given the lack of CO2 effects on RSR, larger amounts of OAexuded per unit root biomass suggest a greater proportional C investment into exudation under eCO2 for this species.
Effect of water treatment on exudation
For E. tereticornis, the water treatment did not change the OA amounts in root exudates, despite higher total biomass and lower RSR for the low-watered plants. Together with slightly smaller amounts of root exudates per unit root biomass, this suggests that E. tereticornis seedlings invested less C in OAs or belowground biomass and more C in aboveground growth under low-watered conditions than high-watered conditions. This may be related to its survival strategy at an early phase of juvenile seedling development when it is typically exposed to intense competition for light with surrounding herbaceous species on the forest floor (Collins et al. 2018).
Hakea sericea, on the other hand, had greater total amounts of OAexuded per plant and lower total OArhizo amount per unit root mass under low-watered conditions relative to high-watered conditions. While this species allocated more biomass belowground under low-water conditions (increased total biomass and RSR), the number of cluster roots per unit root biomass decreased. These results suggest that H. sericea invested C into developing non-cluster roots under low-watered conditions, and its larger root system could maintain similar whole plant OAexuded levels under both watering regimes.
Higher plant growth for M. stipoides under low-watered than high-watered conditions did not increase the total amount of OAs exudated, as they were counterbalanced by smaller amounts per unit root biomass. Thus, M. stipoides did not invest more C into exudation under low-watered conditions, despite greater plant growth.
Effect of CO2 and water treatments on OA composition in association with plant/soil measurements
CO2 concentrations and water availability altered OA composition for E. tereticornis and M. stipoides but not for H. sericea, mainly via changes in malic acid, accompanied by changes in soil phosphate concentrations. Given the positive relationship between the total OArhizo amount for M. stipoides (but not E. tereticornis) and soil phosphate, with malic acid the primary OA for this species, these results suggest that M. stipoides exposed to eCO2 (or low-watered conditions) secretes a relatively larger (or smaller) quantity of malic acid in the rhizosphere, facilitating (or suppressing) soil P mobilisation (Bais et al. 2006; Dakora and Phillips 2002; Ryan et al. 2001). Additional (or alternative) explanations for decreased soil P availability at low-watered conditions are that greater plant growth, and therefore P uptake, reduced available P and/or that low water availability restricted P mobility in the soil. Despite E. tereticornis increasing the amount of malic acid exuded in the rhizosphere in response to eCO2, it did not change the total OArhizo amount or soil phosphate concentration. Furthermore, notwithstanding the higher P mobilisation efficiency of oxalic than malic acids (on a molar concentration basis) (Mendes et al. 2020), the increased amount of oxalic acid in OArhizo in low-watered E. tereticornis plants co-occurred with decreased soil phosphate concentrations. While many hydroponic studies have demonstrated increased rates of root exudation at low P supply to facilitate P uptake (e.g. Khorassani et al. 2011), responses differ among organic compound types. For instance, O’Sullivan et al. (2021) reported increased rates of citric and malic acids in response to eCO2 and low P supply in white lupin while fumaric acid was unaltered. In our study, CO2 and water treatment did not influence the C allocation of E. tereticornis to root exudation but shifted OA composition; however, this did not seem to facilitate P mobilisation. The relationship between particular OAs and soil P availability is highly species-specific, especially when using soil from a natural ecosystem.
Estimating C fluxes from root exudates of OAs in a Eucalyptus woodland under eCO2
Although it varies substantially among plant species, roots secrete approximately 11–40% of assimilated C (Badri and Vivanco 2009; Jones et al. 2009), with OAs accounting for a significant fraction (Jones 1998). For instance, OAs are responsible for 16–31% C in root exudates of maize (Gransee and Wittenmayer 2000). Hence, changes in the exudation rates of OAs could significantly change the amount of C secreted from plant roots to soil. Our study provides empirical estimates of the amounts of C secreted from the studied plant species in the form of OAs. Our study was motivated by the EucFACE experiment where M. stipoides is responsible for ~ 99% of the understorey vegetation biomass (Collins et al. 2018; Hasegawa et al. 2018). Given that the soil and seeds of M. stipoides used in this study were collected from this site, the figures obtained from M. stipoides may be relatively realistic estimates of C secreted from roots under field conditions, while those for E. tereticornis and H. sericea may not be comparable to those in the field as they were juvenile seedlings when harvested. If we focus on the exudation of M. stipoides, we can derive estimates of belowground C inputs from the grassy understorey: OArhizo amounts were 4.9 ± 1.6 (aCO2) and 9.0 ± 1.7 (eCO2) µg C plant–1 and OAexuded amounts were 2.6 ± 1.2 (aCO2) and 5.6 ± 1.4 (eCO2) µg C plant–1 h–1, with average aboveground biomass of 0.3 ± 0.03 and 0.4 ± 0.04 g plant–1, respectively (mean ± 1 standard error). Collins et al. (2018) estimated the aboveground biomass of the understorey at EucFACE to be ~ 329 g m–2. Using this value, we can approximate that M. stipoides secretes 4.5 ± 1.3 (aCO2) and 9.8 ± 2.5 (eCO2) mg C m–2 in the rhizosphere and 2.1 ± 0.9 (aCO2) and 4.8 ± 1.0 (eCO2) mg C m–2 h–1 from roots at the EucFACE site. The annual gross primary productivity in the understorey estimated from M. stipoides is 497 and 552 g C m–2 year–1 in aCO2 and eCO2 plots, respectively (Jiang et al. 2020). Thus, M. stipoides roots may have transferred a non-negligible fraction of assimilated C into this system’s soil in the form of OAs. At EucFACE, low soil P availability constrained the positive CO2 effects on the overstorey growth of E. tereticornis (Ellsworth et al. 2017), whereas M. stipoides increased its dominance after three years of CO2 fumigation (Hasegawa et al. 2018). Given that M. stipoides dominated the understorey coverage of this site, it may have contributed to eCO2-induced increases in soil P (as reported in Hasegawa et al. (2016)) via qualitative and/or quantitative changes in root exudates and further supported its growth stimulation by eCO2.
It is often argued that additional C investment into mycorrhizae through root exudation at eCO2 facilitates plant nutrient uptake via enhanced SOM decomposition (priming) (Terrer et al. 2021). However, priming or enhanced mycorrhizal activity may not be the driver of the increased P availability in this system because neither mycorrhizal biomass (both arbuscular- and ecto-mycorrhizae), nor extracellular phosphatase activity, were altered by the CO2 treatment (Castañeda-Gómez et al. 2021). Castañeda-Gómez et al. (2022) further explored the role of arbuscular mycorrhizae in SOM decomposition and P uptake by M. stipoides under eCO2 conditions with a pot study, demonstrating that eCO2 did not alter SOM decomposition regardless of the presence of arbuscular mycorrhizal fungi. Thus, the increased P availability at eCO2 observed at the EucFACE may be derived from inorganic P liberated by OAs from chemical bindings with cations or mineral surfaces and not from the decomposition of organic P by mycorrhizal fungi.
Conclusion
We found that CO2 and water treatments influenced OA amount and composition via increased plant biomass. In general, bigger plants within a species had greater amounts of exudates. When expressed per unit root biomass, OA amounts increased in response to eCO2 for M. stipoides, and low-watered H. sericea plants exuded lower amounts of OAs in the rhizosphere than high-watered plants. Compositional changes in OAs driven by altered exudation of malic acid were associated with changes in soil P availability, possibly explaining the decreased P availability under low-watered conditions and increased P at eCO2 for M. stipoides (but not E. tereticornis). Together with previous studies reporting eCO2-induced increases in soil phosphate availability and soil C fluxes at the EucFACE experiment, our study suggests that changes in root exudation of OAs in M. stipoides could significantly contribute to soil nutrient and C cycling in this Eucalyptus woodland. Thus, increased CO2 concentrations and drastic changes in precipitation patterns (and resultant soil water availability) will likely influence OA amount and composition of the studied Australian native species in low P soil. Quantitative and qualitative changes in root exudates can alter abiotic and biotic interactions in the rhizosphere (de Vries et al. 2019; Drake et al. 2013) and modify edaphic structure by influencing soil aggregates and organo-mineral compounds (Bronick and Lal 2005; Keiluweit et al. 2015), such that the observed changes in OAs in root exudates in this study can have cascading impacts on ecosystem functioning.
Data availability
The raw data and the R scripts that reproduce the results presented in this paper are available to download from Zenodo: https://doi.org/10.5281/zenodo.7522277.
References
Atwell BJ, Henery ML, Rogers GS, Seneweera SP, Treadwell M, Conroy JP (2007) Canopy development and hydraulic function in Eucalyptus tereticornis grown in drought in CO2-enriched atmospheres. Funct Plant Biol 34(12):1137–1149. https://doi.org/10.1071/FP06338
Aulakh MS, Wassmann R, Bueno C, Kreuzwieser J, Rennenberg H (2001) Characterization of root exudates at different growth stages of ten rice (Oryza sativa L.) cultivars. Plant Biol 3(2):139–148. https://doi.org/10.1055/s-2001-12905
Badri DV, Vivanco JM (2009) Regulation and function of root exudates. Plant Cell Environ 32(6):666–681. https://doi.org/10.1111/j.1365-3040.2009.01926.x
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Ann Rev Plant Biol 57(1):233–266. https://doi.org/10.1146/annurev.arplant.57.032905.105159
Bannerman SM, Hazelton PA (1990) Soil landscapes of the Penrith 1:100,000 sheet map and report. Soil Conservation Service of NSW, Sydney
Barton C, Ellsworth DS, Medlyn BE, Duursma RA, Tissue DT, Adams A, Eamus D, Conroy JP, McMurtrie RE, Parsby J, Linder S (2010) Whole-tree chambers for elevated atmospheric CO2 experimentation and tree scale flux measurements in south-eastern Australia: The Hawkesbury Forest Experiment. Agric for Meteorol 150:941–951. https://doi.org/10.1016/j.agrformet.2010.03.001
Bassirirad H, Tissue DT, Reynolds JF, Chapin FS (1996) Response of Eriophorum Vaginatum to CO2 enrichment at different soil temperatures: Effects on growth, root respiration and PO43− uptake kinetics. New Phytol 133(3):423–430. https://doi.org/10.1111/j.1469-8137.1996.tb01909.x
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48. https://doi.org/10.18637/jss.v067.i01
Boudarga K, Lapeyrie F, Dexheimer J (1990) A technique for dual vesicular–arbuscular endomycorrhizal/ectomycorrhizal infection of Eucalyptus in vitro. New Phytol 114(1):73–76. https://doi.org/10.1111/j.1469-8137.1990.tb00375.x
Bronick CJ, Lal R (2005) Soil structure and management: A review. Geoderma 124(1):3–22. https://doi.org/10.1016/j.geoderma.2004.03.005
Calvo OC, Franzaring J, Schmid I, Fangmeier A (2019) Root exudation of carbohydrates and cations from barley in response to drought and elevated CO2. Plant Soil 1–16. https://doi.org/10.1007/s11104-019-03998-y
Campbell CD, Sage RF (2002) Interactions between atmospheric CO2 concentration and phosphorus nutrition on the formation of proteoid roots in white lupin (Lupinus albus L.). Plant Cell Environ 25:1051–1059. https://doi.org/10.1046/j.1365-3040.2002.00883.x
Castañeda-Gómez L, Powell JR, Ellsworth DS, Pendall E, Carrillo Y (2021) The influence of roots on mycorrhizal fungi, saprotrophic microbes and carbon dynamics in a low-phosphorus Eucalyptus forest under elevated CO2. Funct Ecol 35(9):2056–2071. https://doi.org/10.1111/1365-2435.13832
Castañeda-Gómez L, Powell JR, Pendall E, Carrillo Y (2022) Phosphorus availability and arbuscular mycorrhizal fungi limit soil C cycling and influence plant responses to elevated CO2 conditions. Biogeochemistry 160(1):69–87. https://doi.org/10.1007/s10533-022-00939-3
Cawthray GR (2003) An improved reversed-phase liquid chromatographic method for the analysis of low-molecular mass organic acids in plant root exudates. J Chromatogr A 1011:233–240. https://doi.org/10.1016/s0021-9673(03)01129-4
Chilvers GA, Lapeyrie FF, Horan DP (1987) Ectomycorrhizal vs endomycorrhizal fungi within the same root system. New Phytol 107(2):441–448. https://doi.org/10.1111/j.1469-8137.1987.tb00195.x
Collins L, Bradstock RA, de Dios RV, Duursma RA, Velasco S, Boer MM (2018) Understorey productivity in temperate grassy woodland responds to soil water availability but not to elevated CO2. Glob Change Biol 24(6):2366–2376. https://doi.org/10.1111/gcb.14038
Crawley MJ (2012) The R Book, 2nd edn. Wiley, Chichester
Crous KY, Ósvaldsson A, Ellsworth DS (2015) Is phosphorus limiting in a mature Eucalyptus woodland? Phosphorus fertilisation stimulates stem growth. Plant Soil 391:293–305. https://doi.org/10.1007/s11104-015-2426-4
Dakora FD, Phillips DA (2002) Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 245(1):35–47. https://doi.org/10.1023/A:1020809400075
de Vries FT, Williams A, Stringer F, Willcocks R, McEwing R, Langridge H, Straathof AL (2019) Changes in root-exudate-induced respiration reveal a novel mechanism through which drought affects ecosystem carbon cycling. New Phytol 224(1):132–145. https://doi.org/10.1111/nph.16001
Dijkstra FA, Pendall E, Morgan JA, Blumenthal DM, Carrillo Y, LeCain DR, Follett RF, Williams DG (2012) Climate change alters stoichiometry of phosphorus and nitrogen in a semiarid grassland. New Phytol 196:807–815. https://doi.org/10.1111/j.1469-8137.2012.04349.x
Dinkelaker B, Hengeler C, Marschner H (1995) Distribution and function of proteoid roots and other root clusters. Botanica Acta 108(3):183–200. https://doi.org/10.1111/j.1438-8677.1995.tb00850.x
Drake JE, Darby BA, Giasson MA, Kramer MA, Phillips RP, Finzi AC (2013) Stoichiometry constrains microbial response to root exudation. Insights from a model and a field experiment in a temperate forest. Biogeosciences 10(2):821–838. https://doi.org/10.5194/bg-10-821-2013
Drake JE, Aspinwall MJ, Pfautsch S, Rymer PD, Reich PM, Smith RA, Crous KY, Tissue DT, Ghannoum O, Tjoelker MG (2015) The capacity to cope with climate warming declines from temperate to tropical latitudes in two widely distributed Eucalyptus species. Glob Change Biol 21(1):459–472. https://doi.org/10.1111/gcb.12729
Eissenstat DM, Kucharski JM, Zadworny M, Adams TS, Koide RT (2015) Linking root traits to nutrient foraging in arbuscular mycorrhizal trees in a temperate forest. New Phytol 208(1):114–124. https://doi.org/10.1111/nph.13451
Ellsworth DS, Anderson IC, Crous KY, Cooke J, Drake JE, Gherlenda AN, Gimeno TE, Macdonald CA, Medlyn BE, Powell JR, Tjoelker MG, Reich PB (2017) Elevated CO2 does not increase Eucalypt forest productivity on a low-phosphorus soil. Nat Clim Chang 7(4):279–282. https://doi.org/10.1038/nclimate3235
Fox J, Weisberg S (2011) An R companion to applied regression, 2nd edn. SAGE Publications Inc, Thousand Oaks
Ghannoum O, Phillips NG, Conroy JP, Smith RA, Attard RD, Woodfield R, Logan BA, Lewis JD, Tissue DT (2010) Exposure to preindustrial, current and future atmospheric CO2 and temperature differentially affects growth and photosynthesis in Eucalyptus. Glob Change Biol 16(1):303–319. https://doi.org/10.1111/j.1365-2486.2009.02003.x
Gransee A, Wittenmayer L (2000) Qualitative and quantitative analysis of water-soluble root exudates in relation to plant species and development. J Plant Nutr Soil Sci 163(4):381–385. https://doi.org/10.1002/1522-2624(200008)163:4%3c381::AID-JPLN381%3e3.0.CO;2-7
Grose MR, Narsey S, Delage FP, Dowdy AJ, Bador M, Boschat G, Chung C, Kajtar JB, Rauniyar S, Freund MB, Lyu K, Rashid H, Zhang X, Wales S, Trenham C, Holbrook NJ, Cowan T, Alexander L, Arblaster JM, Power S (2020) Insights from CMIP6 for Australia’s future climate. Earths Future 8(5):e2019EF001469. https://doi.org/10.1029/2019EF001469
Hasegawa S, Macdonald CA, Power SA (2016) Elevated carbon dioxide increases soil nitrogen and phosphorus availability in a phosphorus-limited Eucalyptus woodland. Glob Change Biol 22(4):1628–1643. https://doi.org/10.1111/gcb.13147
Hasegawa S, Piñeiro J, Ochoa-Hueso R, Haigh AM, Rymer PD, Barnett KL, Power SA (2018) Elevated CO2 concentrations reduce C4 cover and decrease diversity of understorey plant community in a Eucalyptus woodland. J Ecol 106(4):1483–1494. https://doi.org/10.1111/1365-2745.12943
Helmisaari H-S, Ostonen I, Lõhmus K, Derome J, Lindroos A-J, Merilä P, Nöjd P (2009) Ectomycorrhizal root tips in relation to site and stand characteristics in Norway spruce and Scots Pine stands in Boreal Forests. Tree Physiol 29(3):445–456. https://doi.org/10.1093/treephys/tpn042
Hill JO, Simpson RJ, Ryan MH, Chapman DF (2010) Root hair morphology and mycorrhizal colonisation of pasture species in response to phosphorus and nitrogen nutrition. Crop Past Sci 61(2):122–131. https://doi.org/10.1071/CP09217
Hovenden MJ, Newton PCD, Carran RA, Theobald P, Wills KE, Vander Schoor JK, Williams AL, Osanai Y (2008) Warming prevents the elevated CO2-induced reduction in available soil nitrogen in a temperate, perennial grassland. Glob Change Biol 14(5):1018–1024. https://doi.org/10.1111/j.1365-2486.2008.01558.x
Imas P, Bar-Yosef B, Kafkafi U, Ganmore-Neumann R (1997) Release of carboxylic anions and protons by tomato roots in response to ammonium nitrate ratio and pH in nutrient solution. Plant Soil 191:27–34. https://doi.org/10.1023/A:1004214814504
Jiang M, Medlyn BE, Drake JE, Duursma RA, Anderson IC, Barton CVM, Boer MM, Carrillo Y, Castañeda-Gómez L, Collins L, Crous KY, De Kauwe MG, dos Santos BM, Emmerson KM, Facey SL, Gherlenda AN, Gimeno TE, Hasegawa S, Johnson SN, Kännaste A, Macdonald CA, Mahmud K, Moore BD, Nazaries L, Neilson EHJ, Nielsen UN, Niinemets U, Noh NJ, Ochoa-Hueso R, Pathare VS, Pendall E, Pihlblad J, Piñeiro J, Powell JR, Power SA, Reich PB, Renchon AA, Riegler M, Rinnan R, Rymer PD, Salomón RL, Singh BK, Smith B, Tjoelker MG, Walker JKM, Wujeska-Klause A, Yang J, Zaehle S, Ellsworth DS (2020) The fate of carbon in a mature forest under carbon dioxide enrichment. Nature 580(7802):227–231. https://doi.org/10.1038/s41586-020-2128-9
Jin J, Tang C, Sale P (2015) The impact of elevated carbon dioxide on the phosphorus nutrition of plants: A review. Ann of Bot 116(6):987–999. https://doi.org/10.1093/aob/mcv088
Johnson DW, Cheng W, Joslin JD, Norby RJ, Edwards NT, Todd DE (2004) Effects of elevated CO2 on nutrient cycling in a Sweetgum plantation. Biogeochem 69(3):379–403. https://doi.org/10.1023/B:BIOG.0000031054.19158.7c
Jones DL (1998) Organic acids in the rhizosphere – a critical review. Plant Soil 205(1):25–44. https://doi.org/10.1023/A:1004356007312
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: Carbon trading at the soil–root interface. Plant Soil 321(1):5–33. https://doi.org/10.1007/s11104-009-9925-0
Keiluweit M, Bougoure JJ, Nico PS, Pett-Ridge J, Weber PK, Kleber M (2015) Mineral protection of soil carbon counteracted by root exudates. Nat Clim Chang 5(6):588–595. https://doi.org/10.1038/nclimate2580
Kenward MG, Roger JH (1997) Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53(3):983–997. https://doi.org/10.2307/2533558
Khorassani R, Hettwer U, Ratzinger A, Steingrobe B, Karlovsky P, Claassen N (2011) Citramalic acid and salicylic acid in sugar beet root exudates solubilize soil phosphorus. BMC Plant Biol 11(1):121. https://doi.org/10.1186/1471-2229-11-121
Kidd DR, Ryan MH, Hahne D, Haling RE, Lambers H, Sandral GA, Simpson RJ, Cawthray GR (2018) The carboxylate composition of rhizosheath and root exudates from twelve species of grassland and crop legumes with special reference to the occurrence of citramalate. Plant Soil 424:389–403. https://doi.org/10.1007/s11104-017-3534-0
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) LmerTest package: Tests in linear mixed effects models. J Stat Softw 82(1):1–26. https://doi.org/10.18637/jss.v082.i13
Kuzyakov Y (2010) Priming effects: Interactions between living and dead organic matter. Soil Biol Biochem 42(9):1363–1671. https://doi.org/10.1016/j.soilbio.2010.04.003
Lambers H, Raven JA, Shaver GR, Smith SE (2008) Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol 23:95–103. https://doi.org/10.1016/j.tree.2007.10.008
Lambers H, Bishop JG, Hopper SD, Laliberté E, Zúñiga-Feest A (2012) Phosphorus-mobilization ecosystem engineering: The roles of cluster roots and carboxylate exudation in young p-limited ecosystems. Ann Bot 110:329–348. https://doi.org/10.1093/aob/mcs130
Legendre L, Legendre P (2012) Numerical ecology. Elsevier, Amsterdam
Mendes GO, Murta HM, Vasconcelos Valadares R, Batista da Silveira W, Ribeiro da Silva I, Dutra Costa M (2020) Oxalic acid is more efficient than sulfuric acid for rock phosphate solubilization. Miner Eng 155:106458. https://doi.org/10.1016/j.mineng.2020.106458
Neumann G, Massonneau A, Martinoia E, Römheld V (1999) Physiological adaptations to phosphorus deficiency during proteoid root development in white lupin. Planta 208(3):373–382. https://doi.org/10.1007/s004250050572
Ngugi MR, Hunt MA, Doley D, Ryan P, Dart P (2003) Dry matter production and allocation in Eucalyptus cloeziana and Eucalyptus argophloia seedlings in response to soil water deficits. New for 26(2):187–200. https://doi.org/10.1023/A:1024493917483
O’Sullivan JB, Plozza T, Stefanelli D, Jin J, Tang C (2021) Elevated CO2 and phosphorus deficiency interactively enhance root exudation in Lupinus albus L. Plant Soil 465(1):229–243. https://doi.org/10.1007/s11104-021-04991-0
Ochoa-Hueso R, Hughes J, Delgado-Baquerizo M, Drake JE, Tjoelker MG, Piñeiro J, Power SA (2017) Rhizosphere-driven increase in nitrogen and phosphorus availability under elevated atmospheric CO2 in a mature Eucalyptus woodland. Plant Soil 416:283–295. https://doi.org/10.1007/s11104-017-3212-2
Oksanen J, Guillaume Blanchet F, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2020) Vegan: Community Ecology Package. R package version 2.5-7. https://CRAN.Rproject.org/package=vegan
Ostonen I, Helmisaari H-S, Borken W, Tedersoo L, Kukumägi M, Bahram M, Lindroos A-J, Nöjd P, Uri V, Merilä P, Asi E, Lõhmus K (2011) Fine root foraging strategies in Norway Spruce forests across a European climate gradient. Glob Change Biol 17(12):3620–3632. https://doi.org/10.1111/j.1365-2486.2011.02501.x
Pathare VS, Crous KY, Cooke J, Creek D, Ghannoum O, Ellsworth DS (2017) Water availability affects seasonal CO2-induced photosynthetic enhancement in herbaceous species in a periodically dry woodland. Glob Change Biol 23(12):5164–5178. https://doi.org/10.1111/gcb.13778
Phillips RP, Finzi AC, Bernhardt ES (2011) Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol Lett 14(2):187–194. https://doi.org/10.1111/j.1461-0248.2010.01570.x
Piñeiro J, Pathare V, Ochoa-Hueso R, Carrillo Y, Power SA (2021) No CO2 fertilization effect on plant growth despite enhanced rhizosphere enzyme activity in a low phosphorus soil. Plant Soil 471:359–374. https://doi.org/10.1007/s11104-021-05175-6
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reich PB, Hobbie SE, Lee T, Ellsworth DS, West JB, Tilman D, Knops JMH, Naeem S, Trost J (2006) Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 440:922–925. https://doi.org/10.1038/nature04486
Robredo A, Pérez-López U, de la Maza HS, González-Moro B, Lacuesta M, Mena-Petite A, Muñoz-Rueda A (2007) Elevated CO2 alleviates the impact of drought on barley improving water status by lowering stomatal conductance and delaying its effects on photosynthesis. Environ and Exp Bot 59(3):252–263. https://doi.org/10.1016/j.envexpbot.2006.01.001
Roelofs RFR, Rengel Z, Cawthray GR, Dixon KW, Lambers H (2001) Exudation of carboxylates in Australian Proteaceae: Chemical composition. Plant Cell Environ 24(9):891–904. https://doi.org/10.1046/j.1365-3040.2001.00741.x
Ryan PR, Delhaize E, Jones DL (2001) Function and mechanism of organic anion exudation from plant roots. Annu Rev Plant Physiol Plant Mol Biol 52(1):527–560. https://doi.org/10.1146/annurev.arplant.52.1.527
Ryan MH, Tibbett M, Edmonds-Tibbett T, Suriyagoda LDB, Lambers H, Cawthray GR, Pang J (2012) Carbon trading for phosphorus gain: The balance between rhizosphere carboxylates and arbuscular mycorrhizal symbiosis in plant phosphorus acquisition. Plant Cell Environ 35(12):2170–2180. https://doi.org/10.1111/j.1365-3040.2012.02547.x
Shane MW, de Vos M, de Roock S, Cawthray GR, Lambers H (2003) Effects of external phosphorus supply on internal phosphorus concentration and the initiation, growth and exudation of cluster roots in Hakea Prostrata R.Br. Plant Soil 248(1):209–219. https://doi.org/10.1023/A:1022320416038
Sharma M, Pang J, Wen Z, De Borda A, Kim HS, Liu Y, Lambers H, Ryan MH, Siddique KHM (2021) A significant increase in rhizosheath carboxylates and greater specific root length in response to terminal drought is associated with greater relative phosphorus acquisition in chickpea. Plant Soil 460(1):51–68. https://doi.org/10.1007/s11104-020-04776-x
Shimono H, Konno T, Sakai H, Sameshima R (2012) Interactive effects of elevated atmospheric CO2 and waterlogging on vegetative growth of soybean (Glycine max (L.) Merr.). Plant Prod Sci 15(3):238–245. https://doi.org/10.1626/pps.15.238
Sun Q, Zhang X, Zwiers F, Westra S, Alexander LV (2021) A global, continental, and regional analysis of changes in extreme precipitation. J Clim 34(1):243–258. https://doi.org/10.1175/JCLI-D-19-0892.1
Suriyagoda LDB, Ryan MH, Renton M, Lambers H (2010) Multiple adaptive responses of Australian native perennial legumes with pasture potential to grow in phosphorus- and moisture-limited environments. Ann Bot 105(5):755–767. https://doi.org/10.1093/aob/mcq040
Suriyagoda LBD, Tibbett M, Edmonds-Tibbett T, Cawthray GR, Ryan MH (2016) Poor regulation of phosphorus uptake and rhizosphere carboxylates in three phosphorus-hyperaccumulating species of Ptilotus. Plant Soil 402(1):145–158. https://doi.org/10.1007/s11104-015-2784-y
Terrer C, Vicca S, Hungate BA, Phillips RP, Prentice IC (2016) Mycorrhizal association as a primary control of the CO2 fertilization effect. Science 353(6294):72–74. https://doi.org/10.1126/science.aaf4610
Terrer C, Phillips RP, Hungate BA, Rosende J, Pett-Ridge J, Craig ME, van Groenigen KJ, Keenan TF, Sulman BN, Stocker BD, Reich PB, Pellegrini AFA, Pendall E, Zhang H, Evans RD, Carrillo Y, Fisher JB, Van Sundert K, Vicca S, Jackson RB (2021) A trade-off between plant and soil carbon storage under elevated CO2. Nature 591(7851):599–603. https://doi.org/10.1038/s41586-021-03306-8
Uloth MB, You MP, Cawthray G, Barbetti MJ (2015) Temperature adaptation in isolates of Sclerotinia sclerotiorum affects their ability to infect Brassica carinata. Plant Pathol 64:1140–1148. https://doi.org/10.1111/ppa.12338
van Hees P, Jones DL, Finlay R, Godbold DL, Lundström US (2005) The carbon we do not see—the impact of low molecular weight compounds on carbon dynamics and respiration in forest soils: A review. Soil Biol Biochem 37:1–13. https://doi.org/10.1016/j.soilbio.2004.06.010
Walker TW, Syers JK (1976) The fate of phosphorus during pedogenesis. Geoderma 15:1–19. https://doi.org/10.1016/0016-7061(76)90066-5
Walker AP, De Kauwe MG, Bastos A, Belmecheri S, Georgiou K, Keeling RF, McMahon SM, Medlyn BE, Moore DJP, Norby RJ, Zaehle S, Anderson-Teixeira KJ, Battipaglia G, Brienen RJW, Cabugao KG, Cailleret M, Campbell E, Canadell JG, Ciais P, Craig ME, Ellsworth DS, Farquhar GD, Fatichi S, Fisher JB, Frank DC, Graven H, Gu L, Haverd V, Heilman K, Heimann M, Hungate BA, Iversen CM, Joos F, Jiang M, Keenan TF, Knauer J, Körner C, Leshyk VO, Leuzinger S, Liu Y, MacBean N, Malhi Y, McVicar TR, Penuelas J, Pongratz J, Powell AS, Riutta T, Sabot MEB, Schleucher J, Sitch S, Smith WK, Sulman B, Taylor B, Terrer TMS, Treseder KK, Trugman AT, Trumbore SE, van Mantgem PJ, Voelker SL, Whelan ME, Zuidema PA (2021) Integrating the evidence for a terrestrial carbon sink caused by increasing atmospheric CO2. New Phytol 229(5):2413–2445. https://doi.org/10.1111/nph.16866
Zhou W, Zhao D, Lin X (1997) Effects of waterlogging on nitrogen accumulation and alleviation of waterlogging damage by application of nitrogen fertilizer and Mixtalol in winter rape (Brassica napus L.). J Plant Growth Regul 16(1):47–53. https://doi.org/10.1007/PL00006974
Acknowledgements
We thank Greg Cawthray for his help with the root exudate analysis, Renee Smith for managing the glasshouse, Gavin McKenzie for managing the laboratory, Pushpinder Matta and Christopher Mitchell for help with nutrient analyses, Burhan Amiji for help with soil sampling and Elise Pendall for help and guidance with setting up the experiment.
Funding
Open access funding provided by Umea University. Shun Hasegawa received Endeavour Scholarships and Fellowships from the Australian Government.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Shun Hasegawa led material preparation, data collection and statistical analysis. Megan H. Ryan led root exudate analysis. The first draft of the manuscript was written by Shun Hasegawa and Sally A. Power, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Honghua He.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hasegawa, S., Ryan, M.H. & Power, S.A. CO2 concentration and water availability alter the organic acid composition of root exudates in native Australian species. Plant Soil 485, 507–524 (2023). https://doi.org/10.1007/s11104-022-05845-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05845-z