Abstract
Purpose
The increase in frequency of freeze–thaw episodes with the diminution of snow cover protection due to climate change compromises the winter survival of alfalfa (Medicago sativa L.). Symbiosis with cold-tolerant rhizobial strains can improve the ability of alfalfa to survive and grow under stressful conditions.
Methods
Six strains of Sinorhizobium (Ensifer) meliloti were tested in combination with two alfalfa populations bred to differ in their levels of freezing tolerance. Plants of each different combination were grown for eight weeks in a growth chamber before being exposed to temperatures promoting their acclimation to cold. Plants were then exposed to a freezing stress (-11ºC) and regrown for three weeks. Shoot, root and nodule biomass were measured before cold acclimation and three weeks after the freezing stress.
Results
After freezing stress, the alfalfa population A-TF7 had shoot and root biomasses that were respectively 19% and 15% larger than cultivar A-TF0. Alfalfa plants inoculated with strain NRG34 showed both a larger shoot biomass and a higher nodule dry weight than plants inoculated with any other strains. Assessment of freezing damages on nodules showed that plants inoculated with NRG34 had the largest proportion of undamaged nodules or of nodules with a regeneration zone.
Conclusion
This study shows for the first time a relationship between nodule and shoot regrowth after a freezing stress, the latter being linked with the proportion of nodules showing less freezing damage. Our results demonstrated that both the choice of alfalfa populations and S. meliloti strains adapted to stress are complementary to increasing alfalfa persistence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alfalfa (Medicago sativa L.), a perennial legume crop, is the most important forage species and the fourth largest crop by area in Canada with 3 million hectares of alfalfa pure stand or alfalfa-based mixtures grown (Statistics Canada 2022). Considered to be the "queen of forages" alfalfa is cultivated worldwide for its high-quality forage (Li and Brummer 2012) and its ability to establish a symbiosis with its nitrogen fixing bacterial partner rhizobia Sinorhizobium (Ensifer) meliloti.
The ability to tolerate low freezing temperatures is a major component of winter survival and field persistence of alfalfa in northern latitudes (Bélanger et al. 2006). Predicted climate changes will likely increase the risks of winter injury to alfalfa in eastern Canada due to higher temperatures in autumn causing less favorable hardening conditions and the diminution of snow cover protection during winter (Bélanger et al. 2002). With the reduction of the snow cover and lack of soil insulation, belowground parts of the plant will be more exposed to freezing temperatures causing winter injuries and mortality (Ambroise et al. 2020). Therefore, the improvement of freezing tolerance will continue to be a crucial issue for the persistence of this species under current and future climatic conditions in northern regions (Bertrand and Castonguay 2013; Castonguay et al. 2006). Recurrent selection is a cyclical breeding method involving repeated exposures of a large number of genotypes to a given stress followed by the selection of genotypes showing tolerance to the stress. The large reservoir of genetic diversity in alfalfa allows development of methods using recurrent selection performed indoors to successfully improve the freezing tolerance and field persistence of alfalfa (Castonguay et al. 2009).
Growing evidence show that the symbiosis between legumes and rhizobia could modify plant physiology and improve tolerance to various abiotic stresses such as drought (Staudinger et al. 2016), high temperatures (Alexandre and Oliveira 2011), salinity (Bertrand et al. 2020a), alkalinity (Song et al. 2017) and acidity (Knežević et al. 2022), thus contributing to their persistence under stressed conditions. A recent study based on 4500 alfalfa plants highlighted the important role played by the rhizobial symbiosis in low temperatures stress tolerance. The study reported that more plants with active nodule survived and showed less cell membrane damage than those with inactive nodules or those without nodules, under the same low temperature-stress condition (-6 °C) (Liu et al. 2019). Because nodule formation is energy demanding, the host plant exerts a tight control on the number of nodules it forms. Inhibition of nodule formation by host plant has been reported in the case of non-efficient rhizobia genotypes or when the nitrogen supply in the soil is sufficient (Regus et al. 2015; Zhang et al. 2020). Soil temperature is a critical factor strongly influencing interactions between plant, soil, and the microbiome; cold temperatures can affect every step of the establishment of the interaction between rhizobia and legumes as well as the efficiency of the rhizobia to fix atmospheric N2 (Alexandre and Oliveira 2013).
The negative effects caused by environmental stresses on the legume-rhizobia symbiosis are well documented and the tolerance of some associations were reported (Bertrand et al. 2015; Sanz-Sáez et al. 2012) but the underlying mechanisms driving these adaptations are not fully characterized (Ferguson et al. 2019). The developmental plasticity of the belowground plant parts in response to abiotic stresses is of key importance for the long-distance transport of substances to assist plant stress resilience (Li et al. 2021). As already mentioned, the host-plant modulates resource allocation to nodules, depending on resources availability and environmental conditions. As a counterpart, the rhizobial partner also exert a control through its sink strength for metabolites to maintain nodule activity (Bertrand et al. 2015, 2016; Marquez-Garcia et al. 2015).
The study of legume-rhizobia symbiosis tolerance to multiple stresses has revealed that biological nitrogen fixation can be improved by the selection of nitrogen-fixing endosymbionts that are well-adapted and tolerant to a broad range of environmental stresses (Atieno and Lesueur 2019). It was shown that rhizobial strains differ in their abilities to nodulate alfalfa at low temperatures (Rice et al. 1995). Furthermore, the level of freezing tolerance and the regrowth of alfalfa after overwintering was influenced by the strains of S. meliloti, showing that the selection of adapted strains could improve plant survival after winter (Bertrand et al. 2007; Prévost et al. 2003). In light of these results, there is a high probability that the interaction between freezing-tolerant alfalfa genotypes and cold-adapted S. meliloti strains will promote field persistence and counteract the negative effect of low temperatures and freezing stress (Prévost et al. 1999). To our knowledge, no studies have compared the impact of different rhizobia strains on the resource allocation to nodules and their impact on regrowth of alfalfa populations differing in their level of freezing tolerance under simulated fall and winter conditions.
The objective of this study was to compare the regrowth of different combinations of S. meliloti strains and alfalfa populations after their exposure to a freezing stress in order to identify the most efficient association strain/population. We hypothesized that alfalfa regrowth after freezing would differ according to the strains used and that better strains could be identified. Twelve associations were compared (six S. meliloti strains with two alfalfa populations) and the detailed phenotyping of aboveground and belowground traits, including nodule damages, were made after the freezing stress to understand the mechanisms of resistance of the associations.
Materials and methods
Characterization of plant material
Two populations of alfalfa (Medicago sativa L.) were used for this study: the cultivar ‘Apica’ (A-TF0) adapted for growth in eastern Canada and developed at the Quebec Research and Development Centre (QRDC) of Agriculture and Agri-Food Canada (Michaud et al. 1983), and A-TF7, a population obtained after seven cycles of recurrent selection for improved freezing tolerance from the original cultivar Apica (Bertrand et al. 2014; Castonguay et al. 2009).
To assess their levels of freezing tolerance and determine if the freezing tolerance of A-TF7 was improved as compared to A-TF0, the two alfalfa populations were cold acclimated under natural winter conditions in an unheated greenhouse and, after 16 weeks of overwintering, cold-acclimated potted plants were transferred into a programmable walk-in freezer set at -2 °C to assess the lethal temperature for 50% of the plants (LT50). The temperatures tested were -2, -14, -16, -18, -20, -22, -24, -26, and -28 °C (6 pots per temperature per populations for a total of 96 pots). After 3 weeks of regrowth, the survival count of the plants at each temperature were recorded and the LT50 was calculated (Bertrand et al. 2020b).
Sinorhizobium meliloti strains and inoculum production
Sinorhizobium meliloti strains ‘A2’, ‘NRG34’, ‘I1’, ‘S27’, and ‘Rm1521’ isolated from different Canadian regions (Table 1) were selected from the AAFC collection for this study based on their nodulation performance (nodule number and color) and alfalfa biomass yield after 8 weeks of growth under low temperature (15/10 °C, day/night) under controlled conditions (D’Amours et al. in preparation). These strains were also characterized for their genetic diversity using multilocus sequence typing (MLST) analysis based on five Sinorhizobium (Ensifer) meliloti housekeeping genes as well as genes related with nodulation capacity and nitrogen fixation, for a total of 18 genes (Rocher et al. in preparation). The choice of strains was also based on previous studies on cold tolerance (A2 and NRG34, Bertrand et al. 2007; Olsen et al. 1994; Rice et al. 1995) and tolerance to other abiotic factors (Rm1521, Bertrand et al. 2015, 2016, 2020a; Bromfield et al. 1994) of S. meliloti strains. The stress tolerant elite strain ‘B399’ (provided by Instituto de Genética “Edwald Alfredo Favret”, INTA, Buenos Aires, Argentina), was also included in the study as a control commercial strain. Strain B399, originally named Rhizobium meliloti ‘102F34’ (Nitragin Co., Milwaukee, WI), has been shown to have a high capacity to fix nitrogen under water-deficit field conditions in symbiosis with different alfalfa cultivars (Jozefkowicz et al. 2017) and under various abiotic stresses including cool temperature and elevated CO2 (Sanz-Sáez et al. 2012).
S. meliloti strains were grown on yeast mannitol agar plates (Vincent 1970). A unique colony was transferred in yeast mannitol broth (YMB, 8-mL per tube) and then placed in a shaking incubator (120 rpm, Lab-Line Orbit Environ-shaker, Melrose Park, IL) at 28 °C for 24 to 48 h. Then 300 µl of each strain were used to inoculate 125 mL of YMB in a 250 ml Erlenmeyer flask and placed in a shaking incubator (200 rpm, Lab-Line Orbit Environ-shaker, Melrose Park, IL) at 28 °C for 24 to 48 h. For each strain, a viability count was performed to adjust inoculum at 108 cells mL−1.
Freezing stress experiment combining contrasting alfalfa populations and cold tolerant Sinorhizobium meliloti strains
Plant growth conditions
Alfalfa seeds of the two populations were surface-sterilized by immersion in ethanol 95% for 30 s, and twice in sodium hypochlorite (3.0% v/v) for 2 min, and then washed three times with sterile distilled water and dried. Three seeds were sown in individual Ray Leach Cone-tainersTM (SC-10 Super Cell. Stuewe & Sons Inc, Tangent, OR) previously washed and sterilized and filled with sterilized Turface® MVP®, a heat-treated montmorillonite clay mineral product (Profile Products LLC, Buffalo Grove, IL).
Plants were grown in growth chambers (Conviron model PGW40, Winnipeg, Canada) under a 21/17 °C day/night temperature regime, a 16-h photoperiod and a photosynthetic photon flux density of 600–800 μmol photons m−2 s−1. Plants were watered once a day during seed germination to avoid Turface dryness. One week after seeding, at the first true trifoliate emergence stage, seedlings were thinned to keep only one plant per cone-tainer and fertilized with 2 mL of sterilized 0.25 N-free Hoagland solution. Each of a total of 384 plants was then inoculated with 1 mL each of the six strains containing 108 cells. For each alfalfa populations (A-TF0 and A-TF7), uninoculated controls were also included to ensure that there was no contamination between tubes but these stunted chlorotic plants were not included in the statistical analysis. For the eight following weeks after inoculation, plants were well-watered daily to keep a constant moisture and 3 mL of sterilized 0.50 N-free Hoagland solution was applied three times a week. During the four weeks following cold acclimation (described below), plants were not fertilized. During the three-week period of regrowth after freezing, plants received 5 mL of sterilized 0.50 N-free Hoagland solution three times a week.
After eight weeks of growth, non-acclimated (NA) plants were collected, and destructive plant measurements were made on 96 plants (8 replicates × 2 alfalfa populations × 6 strains) as described in the next section. Remaining plants were then cold acclimated (CA) for two weeks at 2 °C under 150 µmol m−2 s−1 PPFD and a short photoperiod (8 h) followed by two weeks at -2 °C in the dark to stimulate hardening conditions. Following CA, 96 plants were sampled for measurements after thawing one night at 4 °C. The remaining plants were transferred into a large programmable walking freezer set at -2 °C. Temperature in the freezer was lowered by 3 °C during a 30-min period followed by a 90-min plateau at each of the following temperatures: -5 °C, -8 °C and, finally, -11 °C which was the targeted freezing-stress temperature determined by a preliminary experiment as a damaging, non-lethal temperature for the two alfalfa populations under study. At the end of the 90-min plateau at -11 °C, plants were removed from the freezer and thawed for 24 h at 4 °C in darkness. After thawing, shoots were cut and plants were gradually exposed to the initial optimal regrowth conditions by progressively increasing the air temperature from 4 °C to 21/17 °C day/night in one hour under a 16 h photoperiod with 600–800 µmol photons m−2 s−1 PPFD. A third destructive sampling of 96 plants was made two days after the freezing stress (AFS) to collect information on plant damages immediately following the freezing stress. The last sampling of the remaining 96 plants was made after three weeks of regrowth under the initial environmental conditions (Regrowth after freezing; RAF).
The experiment was assigned to a randomized complete block design with eight blocks and a factorial combination of two alfalfa populations inoculated with the six S. meliloti strains for a total of twelves combination of treatments. Altogether, 384 individual plants (2 populations × 6 strains × 8 repetitions × 4 sampling events) were grown in individual cone-tainers tube which each represented one experimental unit.
Plant measurements
After eight weeks of growth the photosynthetic rate of all non-acclimated-NA plants was measured using a portable photosynthesis system (LI-6400XT, Licor, Inc, Lincoln, NE). Measurements were carried out between 10:00 and 12:00 a.m. and were made on the middle leaflet of the youngest trifoliate fully emerged from the top leaves (one leaflet per plant). Measurements were conducted at the photon flux density of 1000 μmol m−2 s−1, leaf temperature of 23 ± 2 °C and a relative air humidity of 60%. A second measurement of photosynthetic rate was made on the regrowth of alfalfa three weeks after the freezing stress (RAF).
At each sampling event (NA, CA, AFS, and RAF) plants were carefully removed from their cone-tainers and gently shaken to remove the excess of Turface and then washed three times in distilled water. Excess water was removed by gently pressing roots in absorbent paper towels. Nodules were detached from roots with tweezers and placed in 5-mL tubes kept on ice during sampling. After freezing at -80 °C nodules were freezed-dried (Labconco, Model Freezone12, Kansas City, MO) and the dry weight (DW) was recorded. Roots were separated from shoots directly under the crown, and both plant parts were dried separately at 55 °C for 72 h. Dry weight (DW) of nodules, roots and shoots were recorded and used to calculate root:shoot and nodules:root ratios. Shoots were only sampled for NA and RAF plants since CA and AFS shoots and leaves were killed by the 2-wks exposure to subfreezing temperature in the dark.
Plant phenotypic traits and nodules characterization after the freezing stress
For a detailed characterization of nodule regeneration, and root and shoot regrowth after the freezing stress (RAF) the phenotyping of the twelve combinations described previously (2 alfalfa population × 6 strains) was made on five replications for a total of 60 plants.
Each plant was gently removed from the Turface and roots were soaked three times in distilled water and quickly pressed with paper towel to absorb the excess water on roots. Shoot height was measured, and plant vigor was assessed visually using the following scale: 1- Plants very chlorotic (yellow), 2- Plants lightly chlorotic (pale green and yellow), 3- Plants green and relatively small, 5- Plants green and vigorous (Risula 2019). The developmental stage of alfalfa was determined based on the classification of Mueller and Fick (1989) 0-Early vegetative, 1-Mid-Vegetative, 2-Late Vegetative, 3-Early bud, 4- Late bud, 5-Early Flower, 6- Late Flower. Roots were separated from shoots under the crown. Root systems were kept on ice during the characterization of nodulation and nodule sampling. Afterward, shoots and roots were dried at 55 °C for 72 h to record dry weight and to calculate root:shoot ratio.
Nodule characterization was assessed by examining the following features on each root system (60 plants): 1- nodule abundance; 2- nodule position on the root system; 3- nodule height on the root system. The following traits concerning specifically the nodules of each of the 60 plants were also assessed, 4- nodule shape and 5- nodule freezing-damages.
Nodule abundance was recorded as follows, using the modified scoring index of Knight (2007): 0- zero nodules, 1- less than 10 nodules, 2- between 10 and 30 nodules and 3- over 30 nodules. Nodule position was assessed using the three following classes: 1- mostly lateral nodulation only, 2- mostly crown nodulation only and 3- both crown and lateral nodulation (Risula 2019). Nodule height was assessed by visually scoring the percentage of nodule distribution on the following three root section depths starting just below the crown: from 0 to 2 cm, from 2 to 8 cm, and from 8 to 19 cm depth [modified from Bertrand et al. (2020a)]. Nodule shapes were separated into four categories as described for Medicago truncatula (Cai et al. 2018): S; Unbranched and small nodules, E; Unbranched and elongated nodules, B; Bifurcated nodules, PC; Palmate-coralloid nodules. A percentage was attributed for each of these shapes on the 60 plants. For the evaluation of nodule freezing-damages, three classes were used: I; pink nodules presenting no freezing-damages, II; necrotic nodules with pink regeneration zones, III; necrotic nodules. For each plant, the percentage of nodule representing each class was determined visually.
Statistical analysis
To evaluate the freezing tolerance of the two alfalfa populations (A-TF0 and A-TF7), the LT50 was computed with the SAS GENMOD procedure using a probit regression model with soil temperature, population, and their interaction considered as independent variables and mean percent survival as the dependent variable. Differences in LT50 between populations were established using a chi-square goodness of fit test as described in Castonguay et al. (2009).
Photosynthesis and biomass measurements were analyzed using a two-way analysis of variance (ANOVA) model for a randomized complete block design with the SAS MIXED procedure (SAS® Studio, 2020, Version 3.81, SAS Institute Inc., Cary, NC). The model was used to establish the effects of alfalfa populations with contrasted level of freezing tolerance, S. meliloti strains and their interactions, on shoot, root, nodules and total dry weight, root:shoot and nodule:root ratios, photosynthetic rate, plant development stages and plant height. The ANOVA was performed for each sampling events separately. Residual normality and variance homogeneity were verified using the UNIVARIATE procedure. The Shapiro–Wilk’s and Kurtosis’s tests were used to verify the normality of the data distribution. Pairwise comparisons of means differences were made using a Fisher’s least significance difference test (LSD) at P ≤ 0.05 (Figs. 1, 2, 3, 4, and 5).
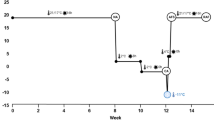
(a) Three-weeks regrowth of the two alfalfa populations under study: original cultivar Apica (A-TF0) and A-TF7 obtained after seven cycles of recurrent selection for improved freezing tolerance within cultivar Apica, after being exposed to the freezing temperatures indicated below each row of pots. (b) Freezing tolerance assessed as the freezing lethal temperature for 50% of the plants (LT50 ± SE) of the two alfalfa populations described above (6 pots per temperature per population for a total of 96 pots). Error bars represent the Standard Error of the Means (SEM). *** indicates that P < 0.001
Shoot and root dry weight of the two alfalfa populations contrasted in their freezing tolerance levels (A-TF0 and A-TF7). Plants grown under controlled conditions were sampled at four physiological stages (48 plants per population for each sampling event): non-acclimated plants (NA) were grown 8 weeks under a 21/17°C, Day/Night (D/N) temperature regime. Plants were then cold acclimated during 2 weeks at 2°C followed by two weeks at -2°C and sampled again (CA). After their exposure to a freezing stress of -11°C, alfalfa plants were transferred back to optimal regrowth conditions (21/17°C, D/N) and sampled after two days (AFS), and after three weeks (RAF). Shoots were only sampled for NA and RAF plants since CA and AFS shoots and leaves were killed by the 2-wks exposure to subfreezing temperature in the dark while root dry weight was measured at the four stages. Error bars represent the SEM, n = 24. Significant differences between populations at each stage, determined by a t-test, are indicated with the following levels of probability: * P ≤ 0.05, ** P < 0.01, *** P < 0.001
Nodule dry weight (DW) and nodule:root DW ratio of the two alfalfa populations with contrasted level of freezing tolerance, A-TF0 and A-TF7 (48 plants per population for each sampling event). Nodules and roots were sampled at the following physiological stages of the plants: non-acclimated (NA), cold-acclimated (CA), two days after the exposure to a freezing stress (AFS) and three weeks regrowth after a freezing stress (RAF). Nodules and roots were freeze-dried, and their dry weight was determined. Error bars represent the SEM, n = 24. Significant differences between populations at each stage are indicated with the following levels of probability: * P ≤ 0.05, ** P < 0.01, *** P < 0.001
(a) Visual representation of the regrowth of each alfalfa population/strain associations 2-wk after a freezing stress of -11 °C. The two alfalfa populations are cultivar Apica (A-TF0) and freezing-tolerant A-TF7 inoculated with one of the six S. meliloti strains (B399, A2, NRG34, S27, Rm1521 and I1. (b) Total dry weight (DW) of the three weeks regrowth of two alfalfa populations with contrasted level of freezing tolerance (A-TF0 in white, A-TF7 in green) averaged across the 6 strains (48 plants per populations). (c) Average shoot DW of the two alfalfa populations inoculated with each of the six strains (16 plants per strain) measured three weeks after the freezing stress. (d) Average shoot:root ratio of the two alfalfa populations inoculated with each of the six strains (16 plants per strain) measured three weeks after the freezing stress. (e) Photosynthetic rates of the two alfalfa populations (A-TF0 in white, A-TF7 in green), inoculated with the six S. meliloti strains (8 plants for each Pop × strain association). Error bars represent the SEM. *** indicates differences in a T-test at P < 0.001. Different letters represent significant differences among strains as determined by the Fisher’s least significant difference (LSD) test at P ≤ 0.05
Nodule dry weight (DW) and nodule:root DW ratio averaged over the two alfalfa populations in response to inoculation with one of the six different S. meliloti strains (B399, A2, NRG34, S27, Rm1521 and I1). Nodules and roots (16 plants for each strain) were sampled at the following sampling events: non-acclimated (NA), cold-acclimated (CA), two days after the exposure to a freezing stress (AFS) and three weeks regrowth after a freezing stress (RAF). Error bars represent the Standard Error of the Means (SEM). Different letters represent significant differences among strains for each sampling events determined by the Fisher’s least significant difference (LSD) test at P ≤ 0.05
Three weeks after the freezing stress, a visual rating of plant vigor (Fig. 6), nodules abundance, position and height on the root system were assessed using the relative frequency of each class or index for each phenotypic trait. The percentage of distribution of nodules within the four shape categories described and of nodule freezing-damages within the three classes described were transformed as reported in Aitchison (1986), as they are not independent. Multivariate analysis of repeated measurements was conducted using the SAS MIXED procedure to establish the effects of alfalfa populations with contrasted level of freezing tolerance, S. meliloti strains, and their interactions on the profile of the percent distribution within the four nodules shapes and the three classes of nodule freezing-damages. A maximum likelihood ratio test was performed for model adjustment. Different variances were obtained for each combination and the model presenting the best fit for the data set was used for the comparison based on strains (Supplemental Tables 4a and 5a). Multiple comparisons between strains were adjusted by Bonferroni’s test at P ≤ 0.05 (Supplemental Tables 4b and 5b) and back-transformed for the presentation of results in Fig. 7.
Visual rating of plant vigor of the two alfalfa populations (A-TF0 and A-TF7, left panel) and in response to inoculation with the six rhizobia strains (right panel) at the regrowth after freezing (RAF) stage. Each plant was rated according to the following classes: 1- Plants very chlorotic (yellow), 2- Plants lightly chlorotic (pale green and yellow), 3- Plants green and relatively small, 4- Plants green and vigorous. The relative frequency distribution of each class was calculated for the two populations (30 plants per population) and in response to inoculation with each strains by compounding the two populations (10 plants per strain)
Nodulation characteristics of alfalfa inoculated with six different S. meliloti strains. The following assessments were made on 60 plants (2 alfalfa population x six strains × 5 repetitions) at the regrowth after freezing (RAF) stage. (a) Illustration of the three classes of nodule location on the root system: 1- mostly lateral nodulation only, 2- mostly crown nodulation only and 3- both crown and lateral nodulation. (b) Relative frequency distribution of the three classes of nodules location in response to the six S. meliloti strains. (c) Inward circles represent the percentage distribution of visible freezing-damages to the nodules according to the three following classes: I; pink nodules with no damage, II; necrotic nodules with regeneration zones, III; necrotic nodules. Color codes are illustrated in the lower panel. Significant differences for distribution of freezing damages between strains are indicated by different letters at the circle center. Outward circles represent the percentage distribution of different nodule shapes associated to the six S. meliloti strains. The four following shape types are illustrated with their color code in the right panel: S; Unbranched and small nodules, E; Unbranched and elongated nodules, B; Bifurcated nodules, PC; Palmate-coralloid nodules. Significant differences in shape distribution between strains are indicated by different letters outside the outward circle. Significant differences were adjusted with Bonferroni’s test at P ≤ 0.05. Since the only significant effect was between strains, results from both alfalfa populations were pooled for n = 10
Results
Freezing tolerance
After seven cycles of recurrent selection (TF7), plant freezing tolerance was significantly improved (P < 0.0001) from a lethal temperature for 50% of the plants (LT50) of -20 °C in the original cultivar Apica (A-TF0) to -26 °C in A-TF7 (Figs. 1a and b).
Freezing stress experiment combining contrasting alfalfa populations and cold tolerant Sinorhizobium meliloti strains
Differential response of alfalfa populations to cold acclimation and freezing stress
After 8 weeks of growth under optimal conditions (NA) we did not observe significant differences in above-ground traits between the two alfalfa populations except for the plant developmental stage (Table 2, Supplemental Table 1) which differed slightly. The development of A-TF0 was slightly ahead (early flower, stage 5) of that of A-TF7 (late bud, stage 4) (data not shown).
After four weeks of cold acclimation (CA) root DW increased by 16% (average for both alfalfa populations) when compared to the non-acclimated (NA) root DW. After two weeks of cold acclimation (CA) and two days of regrowth after the freezing stress (AFS), the two alfalfa populations differed in their root DW, with an average of 10% larger roots DW for A-TF7 (average of 3.3 g per plant) than A-TF0 (average of 3.0 g per plant) (Table 3, Fig. 2). The nodule DW and the nodule:root ratio also differed between populations at AFS, with a larger nodule DW (+ 10%) and nodule:root ratio (+ 24%) being observed for A-TF0 than A-TF7 (Table 3, Fig. 3).
The largest difference between the two alfalfa populations was observed for the plant regrowth three weeks after the freezing stress (RAF). At that stage, total DW (Fig. 4b), as well as shoot DW and root DW (Fig. 2) were all significantly larger for A-TF7 than A-TF0 (Tables 2 and 3). Thus, after freezing, the alfalfa population with highest level of freezing tolerance (A-TF7) averaged 15% more of root DW and 19% more of shoot DW than population A-TF0 (Fig. 2) resulting in 17% more total DW (Fig. 4b). Significant differences at RAF were also observed with 28% higher nodule:root ratio for A-TF0 than A-TF7 (Table 3, Fig. 3).
Differential responses of plants inoculated with S. meliloti strain to cold acclimation and freezing stress
S. meliloti strains did not affect biomass of non-acclimated plants, however, they exerted a significant effect on shoot DW regrowth measured three weeks after the freezing stress (Table 2, Supplemental Table 1). Shoot DWs of plants inoculated with strain NRG34 isolated from Northwestern Canada was significantly larger than that of plants inoculated with all the other strains except for strain S27. Shoot DW of alfalfa inoculated with strain NRG34 was 19% larger than shoot DW of plants inoculated with strains A2 and B399 that had the lowest shoot yields (Fig. 4c). The root:shoot ratio also differed between strains; B399 had the largest root:shoot ratio (1.62), which was significantly higher than all other strains (average 1.33) except for strains A2 that did not differ significantly (Table 2, Fig. 4d). We observed a significant interaction between alfalfa populations and S. meliloti strains for the photosynthetic rate at RAF (Table 2, Supplemental Table 1). While there was no difference between strains for A-TF7, the photosynthetic rates of population A-TF0 varied with strains: the average photosynthetic rate with strains NRG34 and S27 (average of 27.3 µmol CO2 m−2 s−1) was larger than with strain B399 (19.4 µmol CO2 m−2 s−1) and Rm1521 (21.1 µmol CO2 m−2 s−1), while it did not differ significantly than with strains A2, and I1 (Fig. 4e).
Under optimal growth conditions (NA) the nodule DW and nodule:root ratio did not differ between plants inoculated with the different strains. However, nodule DW and the nodule:root ratio differed significantly between strains at the CA, AFS, and RAF sampling events (Table 3, Supplemental Table 2). Overall, the cold acclimation process with low temperature and short photoperiod (CA) increased nodule DW (+ 68%) and the nodule:root ratio (+ 43%) for all plants as compared to NA plants (Fig. 5). Nodule DW was significantly higher on plants inoculated with S. meliloti strains A2, Rm1521 and S27 than on plants inoculated with B399 and I1. Nodule DW on plants inoculated with S. meliloti NRG34 did not differ from the other strains. The nodule:root ratio was significantly higher on plant inoculated with strain A2 and S27 than with B399 and I1, and intermediate with NRG34 and Rm1521 (Fig. 5 upper panel).
A diminution of 22% of nodule DW and of 19% of the nodule:root ratio across all plants compared to CA plant was observed for the sampling event made two days after the freezing stress (AFS) (Fig. 5). The strain effect was similar to the results obtained after CA; plants inoculated with strains B399 and I1 showing the lowest nodule DW and nodule:root ratio while plants inoculated with A2 and NRG34 had the highest nodule DW and nodule:root ratio compared to the other strains (Fig. 5).
A significant effect of S. meliloti strains on nodule DW and nodule:root ratio was observed at the RAF sampling event (Table 3). During the three weeks of plant regrowth after freezing stress, the nodule DW of plants inoculated with strain NRG34 increased markedly. Plants inoculated with strain NRG34 had a significant higher nodule DW (0.078 g) than plants inoculated with all other strains (Fig. 5). Plants inoculated with strain NRG34 showed a + 46% higher nodule DW than the lowest average values associated with plants inoculated with strains S27, Rm1521, B399 and I1 (average of 0.054 g) and + 19% higher DW that plants inoculated with strain A2 (Fig. 5). Moreover, plants inoculated with strain NRG34 presented the highest nodule:root ratio (0.025), significantly higher than those inoculated with strains S27, Rm1521, and I1 while there was no difference between plants inoculated with strain A2 (Fig. 5).
Inoculation with S. meliloti strains significantly affected root DW at the sampling event two days after freezing stress (AFS, Table 3). Root DW was significantly higher on plants inoculated with strain B399 (3.4 g per plant) than for plants inoculated with strains S27 and A2 (average of 2.9 g per plant) while the plants inoculated with the other strains had intermediate root DWs (average of 3.1 g/plant for strains I1, NRG34 and Rm1521) (Supplemental Table 2).
Plant and nodule phenotyping after freezing stress
A detailed phenotyping of plants was made three weeks after the freezing stress (RAF) to assess if some combinations of alfalfa populations and S. meliloti strains had more successful regrowth after freezing. Phenotyping was also conducted to determine which phenotypic traits, particularly regarding nodulation, were associated with more vigorous regrowth.
Shoot regrowth
Shoot DW differed significantly between the two alfalfa populations with the freezing-tolerant population A-TF7 showing a significant larger regrowth than A-TF0 (As reported in Fig. 2 for RAF plants; Supplemental Table 3a). Population A-TF7 presented a larger proportion of ‘green and vigorous’ shoot regrowth (vigor rate of 4) compared to A-TF0 for which 10% of the plants showed a ‘light chlorosis’ (vigor rate of 2, Fig. 6 left panel). S. meliloti strains also exerted a significant effect on shoot DW: plants inoculated with strain NRG34 had a larger shoot DW than plants inoculated with strains A2 and Rm1521 while plants inoculated with strains B399, S27, and I1 had an intermediate shoot regrowth (Supplemental Table 3a). The strain effect was also reflected on the vigour of alfalfa regrowth. Plants inoculated with strain NRG34 showed the largest proportion of ‘green and vigorous’ regrowth (vigor rate 4), followed by I1 and S27, while the regrowth of plants inoculated with B399, A2 and Rm1521 was less vigorous, with a small proportion of ‘lightly chlorotic’ plants of vigor rate 2 (Fig. 6 right panel).
Nodulation characteristics
Nodule location on the root system of alfalfa differed according to the different rhizobial strains under study (Figs. 7a and b). Plants inoculated with strains NRG34 and S27 presented nodulation on both the crown and lateral roots for 100% of the plants sampled. For plants inoculated with strains Rm1521, I1 and B399, all nodules were located on the crown on 5 to 10% of the plants, while for plants inoculated with A2, nodules were evenly distributed on roots with no crown regrouping (Figs. 7a and b).
Percentage of distribution of nodules shapes differed significantly between plants inoculated with each of the strains (Fig. 7c outward circle; Supplemental Tables 3b, 4a and b). Plants inoculated with strain A2 presented the most regular distribution of all nodule shapes with a larger proportion of bifurcated nodules than plants inoculated with the other strains. Plants inoculated with strain NRG34 had the largest proportion of palmate-coralloid nodules than plants inoculated with any other strains while plants inoculated with strains Rm1521, S27, I1 and B399 had intermediate profiles with even proportions of unbranched small nodules, unbranched elongated nodules and palmate-coralloid nodules but low proportion of bifurcated nodules (Fig. 7c outward circle).
The proportion of the three classes of freezing damages on nodules differed significantly between plants inoculated with the six different strains (Fig. 7c inward circle; Supplemental Tables 3b, 5a and b).
Plants inoculated with strain NRG34, showed the largest proportion of pink nodules and nodules presenting a regeneration zone (average of 85%) as compared to plants inoculated with all the other strains except for those inoculated with strain S27 (Fig. 7c inward circle). Plants inoculated with strain A2 had the largest proportion of necrotic nodules (40%) as compared to plants inoculated with all the other strains (Fig. 7c inward circle). The proportion of the three classes of damages were evenly distributed in plants inoculated with strains B399, RM1521, and I1.
Discussion
Freeze–thaw episodes, which are predicted to occur more often under climate change, can be very damaging to alfalfa crops and compromise their persistence. Better understanding of how the symbiosis between the host plant and its rhizobial partner are impacted by cold acclimation and freezing stress is required to elaborate strategies to mitigate the impact of cold stress on alfalfa. In this study, we demonstrated that using alfalfa populations obtained by recurrent selection for freezing tolerance and the use of a tolerant S. meliloti partner are effective and complementary approaches to enhance the persistence of alfalfa exposed to freezing stress.
The assessment of the LT50 of cultivar Apica (A-TF0) and of an alfalfa population obtained after seven cycles of recurrent selection for improved freezing tolerance(A-TF7) revealed a significant increase in freezing tolerance up to -6 °C in response to recurrent selection. Moreover, our results showed that cold-acclimated plants of A-TF7 had a 17% larger biomass regrowth than the initial cultivar A-TF0 following a non-lethal freezing stress of -11 °C. These results are consistent with previous work on the improvement of the freezing tolerance of alfalfa cultivar Apica (Castonguay et al. 2009, 2011) and thus, confirmed the effectiveness of this recurrent selection method performed indoor under control conditions to improve the freezing tolerance of alfalfa and other perennial species (Bertrand et al. 2020b).
One key element of alfalfa persistence is the adequate accumulation of root and crown organic reserves in the form of carbohydrates and amino acids. These reserves are required to sustain the metabolism of overwintering organs and, in the spring, to to support plant regrowth (Bélanger et al. 2006). Although most studies have examined the impact of cold-and freezing-stress on the aboveground parts of plants, the role of belowground organs such as roots and nodules in the cold acclimation process, and their impact on freezing tolerance and plant regrowth have largely been overlooked (Ambroise et al. 2020; Liu et al. 2019; Nieman et al. 2018). Here we clearly documented an increase in root DW of alfalfa following four weeks of cold acclimation at low temperature. Although it is well known that roots hardly grow below 5°C (Zhu et al. 2015), perennial plants have been shown to accumulate carbohydrates and amino acids in roots in response to cold acclimation (Dhont et al. 2002, 2003). This transfer of organic reserves from shoot to roots likely contributed to the increase in root biomass that we observed. The observation of a larger increase in root biomass in population A-TF7 as compared to A-TF0 could explain the superior freezing tolerance of the former population since a larger capacity of translocation of cryoprotective sugars and amino acids to the roots is an adaptation strategy that have previously been reported in recurrently-selected populations when compared to the initial genetic background (Castonguay et al. 2011). In a field study, Dhont et al. (2002) reported a marked increase of alfalfa root biomass with the decline of temperature and photoperiod, concurrently with the accumulation of carbohydrate reserves in belowground organs. The authors also reported a positive correlation between organic reserves in roots and shoot regrowth in the spring in two alfalfa cultivars. Our results show that after three weeks of regrowth after a freezing stress, the difference in root DW was even more pronounced between A-TF7 and A-TF0 than just after cold acclimation showing that the root system is specifically targeted by the method of recurrent selection for improved freezing tolerance that was used. This result also indicates that roots suffered more damages in the less freezing tolerant alfalfa population (Ambroise et al. 2020; Nieman et al. 2018). Future research on plant cold acclimation should include investigations on differential root system architecture and on the impact of root freezing damage on plant regrowth.
The importance of belowground organs in the cold acclimation process of alfalfa is further highlighted by the striking increase in nodule biomass during the four weeks of cold acclimation at low temperature. There is growing evidence that the association between legume-hosts and their rhizobial partners is a key mechanism to increase plant tolerance to various abiotic stresses (Bertrand et al. 2020a; Sanz-Sáez et al. 2012; Sindhu et al. 2020; Song et al. 2017; Staudinger et al. 2016) and to low temperatures stresses in particular (Irshad et al. 2021; Liu et al. 2019; Yuan et al. 2020). Our results showing a large increase of the nodules DW (+ 68%) and of the nodule:root ratio (+ 43%) in response to cold acclimation in alfalfa fully support the importance of this mechanism. Legume hosts have the ability to control the investment in number of nodules by the autoregulation of nodulation (AON) pathway (Ferguson et al. 2010) and environmental factors have been shown to affect the resource allocation process from the plant to the nodules (Friel and Friesen 2019; Goh et al. 2016; Zhang et al. 2020). Marquez-Garcia et al (2015) observed that drought stress induces the senescence of shaded soya leaves before any observation of nodule senescence, suggesting that leaves with a low photosynthetic capacity are sacrificed in favor of nodules for the transfer of nitrogen. Our observation of alfalfa’s allocation of resources to nodules during cold acclimation at low temperature by photosynthates translocation agrees with the observation of Gurusamy et al. (2000), who reported that a substantial amount of starch and lipids were transferred to nodules in perennial beach pea before winter.
Our experiment clearly shows that low temperature triggers the allocation of resources into nodules and that the magnitude of this investment is modulated by the strain of the rhizobial partner since there was no significant effect of the S. meliloti strains when plants were growing under optimal conditions (NA) whereas S. meliloti strains significantly impacted the nodule DW, the nodule root:ratio, and the regrowth alfalfa after cold acclimation as well as following the freezing stress. It seems that legume-host regulates the resource investment in nodules depending on the benefit that the host will further receive from those symbiotic partners (Regus et al. 2015; Westhoek et al. 2021). Our observation that population A-TF0 invests more into nodules after a freezing stress than the more freezing-tolerant population A-TF7 further support this hypothesis. The less freezing-tolerant population (A-TF0) increased the resource allocation in its symbiotic partner by increasing the nodule:root ratio as the benefit of the symbiosis is likely more important for this population than for a more freezing tolerant population suffering less damages. In our study the nitrogen input was only provided by the rhizobia thus highlighting the importance for alfalfa to invest in nodules to ensure its regrowth after the freezing stress.
In response to freezing, we observed different nodule biomass according to the strain of S. meliloti used to inoculate the plants and it could be hypothesized that strains with the largest sink strength are more active after freezing resulting in a larger allocation of cryoprotective compounds such as amino acid, sugars and starch to the nodules, thus increasing their DW. Alfalfa plants under salinity stress conditions have been shown to maintain an active transport from the shoot to the nodules to help maintain nodule activity under stress and it was shown that the sink strength toward nodules was modulated by the level of the salinity tolerance of the strain (Bertrand et al. 2015, 2016). Alfalfa inoculated with strain NRG34, isolated from Northwesthern Canada had larger nodule DW, nodule:root ratio and larger shoot DW than alfalfa inoculated with any other strains. Alfalfa plants inoculated with strain NRG34 also had a better freezing tolerance as showed by their greater shoot weight three weeks following the freezing stress. Plants inoculated with this strain also had a much larger nodule DW than any other strain showing for the first time a strong positive relationship between shoot regrowth and nodule regrowth after a freezing stress. Our results are consistent with previous studies reporting superior adaptation for nodulation and nitrogen fixation at low temperature of strain NRG34 (Rice et al. 1995) and better regrowth potential of overwintering alfalfa inoculated with strain NRG34 (Prévost et al. 2003). However, they are not consistent with the results of Bertrand et al. (2007) who reported superior freezing tolerance of alfalfa cultivar ‘AC Caribou’ inoculated with strain A2 when compared to plants inoculated with strain NRG34. The use of a different genetic background and experimental conditions could explain these different results.
The only statistically significant interaction between the two alfalfa populations and the S. meliloti strains was found for the photosynthetic rate. While there was no difference between strains for the population with the highest level of freezing tolerance (A-TF7), the photosynthetic rate of population A-TF0 varied according to the inoculated strain. Nodules represent a high strength sink and, as such, have been shown to stimulate photosynthetic activity (Concha and Doerner 2020; Kaschuk et al. 2010; Parvin et al. 2020). The allocation of resources to nodules after freezing resulting in a larger nodule:root ratio for population A-TF0 than A-TF7 may explain the higher rates of photosynthesis for alfalfa population A-TF0 since higher photosynthetic rates are directly linked with strain efficiency (Kaschuk et al. 2010). This suggests that the biomass of active nodules with an efficient nitrogen metabolism is important to support plant regrowth after a freezing stress as they stimulate photosynthesis.
To better understand the link between nodulation and more vigorous plant regrowth, we proceeded with a detailed phenotyping of the above and belowground parts of the plants of the different associations between alfalfa populations and strains three weeks after the freezing stress. We observed that alfalfa shoot regrowth and plant vigor were both superior for plants inoculated with strain NRG34 isolated from Northwestern Canada than with the other strains. We noted distinct profile in the percentage of distribution of nodules shapes among the strains. Higher proportion of small unbranched nodule on the roots of plants inoculated by strain NRG34 than with the other strains suggests a higher potential of regeneration of the nodules infected by that strain as those small nodules were all newly formed. Plants inoculated with strains NRG34 and S27 had higher proportions of palmate-coralloid nodules which is the largest size of nodules observed. It could be supposed that palmate-coralloid nodules stored more resources to ensure the regrowth after the freezing stress and represent a strongest carbon sink to sustain the photosynthetic activity (Cai et al. 2018). We also observed a more even distribution of nodules throughout the root system on crowns and lateral roots for plants inoculated with strains NRG34 and S27 suggesting an additional strategy of those strains to infect and colonize the entire root system.
The freezing damage on nodules differed according to the strain in symbiosis with alfalfa, as shown by the different proportions of undamaged nodules, nodules with regeneration zones, and necrotic nodules. The overwintering of nodules has previously been reported for perennial legumes of temperate regions (Bal and Khetmalas 1996; Bergersen et al. 1963; Pate 1961). However, to our knowledge, this is the first report of in vivo differences in nodule freezing damages and regeneration potential induced in legumes by S. meliloti strains. While plants inoculated with strain A2 had the highest nodule DW under optimal growing conditions, they showed the largest proportion of necrotic nodules along with the lowest shoot regrowth three weeks after freezing as compared to the other strains. On the contrary, plants inoculated with strain NRG34, had the largest proportion of active nodules with no damage or with a large regeneration zone after the freezing stress along with the greatest plant vigor and shoot regrowth three weeks after freezing. The positive relationship between less nodule freezing damage and a greater vigorous shoot regrowth reported here opens the door to new strategies to increase legume yield under stress. Taken together, the phenotyping results show that multiple strategies are at play for plants inoculated with strain NRG34 to support a vigorous regrowth after freezing: a capacity to colonize the entire root systems with new active nodules, the presence of large nodules with a strong sink strength, and a higher nodule freezing tolerance.
Conclusion
Our study shows that both plant recurrent selection and selection of cold and freezing tolerant rhizobial strains are complementary and effective approaches to increase the persistence and regrowth of alfalfa exposed to freeze–thaw episodes. The meaningful allocation of resources to nodules by alfalfa plants exposed to cold acclimation conditions highlights the crucial role of the symbiotic partner to increase alfalfa tolerance to stresses. The differential above and belowground responses modulated by the two alfalfa populations shows that different allocation strategies are at play during cold acclimation depending on the level of freezing tolerance of alfalfa.. A detailed plant phenotyping revealed distinct profiles of nodule shapes and different levels of nodules freezing damages depending on the symbiotic S. meliloti strain used. These are the first in vivo observations of the variability of freezing tolerance among S. meliloti strains. These differences also show that plant resource allocation to nodules is modulated by their sink strength and metabolic activity after freezing. Alfalfa regrowth after freezing relies in part on nodule tolerance to freezing stress which in turn depend on S. meliloti strains.
Data availability
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DW:
-
Dry weight
References
Aitchison J (1986) The statistical analysis of compositional data. Monographs on statistics and applied Probability, Chapman & Hall, London
Alexandre A, Oliveira S (2011) Most heat-tolerant rhizobia show high induction of major chaperone genes upon stress. FEMS Microbiol Ecol 75(1):28–36. https://doi.org/10.1111/j.1574-6941.2010.00993.x
Alexandre A, Oliveira S (2013) Response to temperature stress in rhizobia. Crit Rev Microbiol 39(3):219–228. https://doi.org/10.3109/1040841X.2012.702097
Ambroise V, Legay S, Guerriero G, Hausman JF, Cuypers A, Sergeant K (2020) The roots of plant frost hardiness and tolerance. Plant Cell Physiol 61(1):3–20. https://doi.org/10.1093/pcp/pcz196
Atieno M, Lesueur D (2019) Opportunities for improved legume inoculants: enhanced stress tolerance of rhizobia and benefits to agroecosystems. Symbiosis 77:191–205. https://doi.org/10.1007/s13199-018-0585-9
Bal AK, Khetmalas MB (1996) Pre- and postwinter changes in the root nodules of Lathyrus maritimus (L.) Bigel. with special reference to storage organelles. Int J Plant Sci 157:432–439. https://doi.org/10.1086/297359
Bélanger G, Rochette P, Castonguay Y, Bootsma A, Mongrain D, Ryan DAJ (2002) Climate change and winter survival of perennial forage crops in eastern Canada. Agron J 94:1120–1130. https://doi.org/10.2134/agronj2002.1120
Bélanger G, Castonguay Y, Bertrand A, Dhont C, Rochette P, Couture L, Drapeau R, Mongrains D, Chalifour FP, Michaud R (2006) Winter damage to perennial forage crops in eastern Canada: causes, mitigation, and prediction. Can J Plant Sci 86:33–47. https://doi.org/10.4141/P04-171
Bergersen FJ, Hely FW, Costin AB (1963) Overwintering of clover nodules in alpine conditions. Aust J Biol Sci 16:920–921
Bertrand A, Castonguay Y (2013) Molecular changes in recurrently selected populations of forage legumes. In: Yoshida M, Matsumoto N, Imai R (eds) Plant and microbe adaptations to cold in a changing world. Springer, New York, pp 209–217
Bertrand A, Prévost D, Bigras FJ, Castonguay Y (2007) Elevated atmospheric CO2 and strain of rhizobium alter freezing tolerance and cold-induced molecular changes in alfalfa (Medicago sativa L.). Ann Bot 99:275–284. https://doi.org/10.1093/aob/mcl254
Bertrand A, Dhont C, Bipfubusa M, Chalifour FP, Drouin P, Beauchamp CJ (2015) Improving salt stress responses of the symbiosis in alfalfa using salt-tolerant cultivar and rhizobial strain. Appl Soil Ecol 87:108–117. https://doi.org/10.1016/j.apsoil.2014.11.008
Bertrand A, Bipfubusa M, Dhont C, Chalifour FP, Drouin P, Beauchamp CJ (2016) Rhizobial strains exert a major effect on the amino acid composition of alfalfa nodules under NaCl stress. Plant Physiol Biochem 108:344–352. https://doi.org/10.1016/j.plaphy.2016.08.002
Bertrand A, Gatzke C, Bipfubusa M, Lévesque V, Chalifour FP, Claessens A, Rocher S, Tremblay GF, Beauchamp CJ (2020a) Physiological and biochemical responses to salt stress of alfalfa populations selected for salinity tolerance and grown in symbiosis with salt-tolerant rhizobium. Agronomy 10(4):569. https://doi.org/10.3390/agronomy10040569
Bertrand A, Rocher S, Claessens A, Bipfubusa M, Papadopoulos Y, Castonguay Y (2020b) Biochemical and molecular responses during overwintering of red clover populations recurrently selected for improved freezing tolerance. Plant Sci 292:110388. https://doi.org/10.1016/j.plantsci.2019.110388
Bertrand A, Castonguay Y, Bourassa J (2014) A whole-plant screening test to identify genotypes with superior freezing tolerance. In: Hincha, D.K. et Zuther, E. (Eds.), Plant cold Acclimation Methods and protocols. Methods in Molecular Biology 1166, Humana Press, Springer Protocols, Clifton, pp 35–41
Bordeleau LM, Antoun H, Lachance RA (1977) Effets des souches de Rhizobium meliloti et des coupes successives de la luzerne (Medicago sativa) sur la fixation symbiotique d’azote. Can J Plant Sci 57:433–439. https://doi.org/10.4141/cjps77-063
Bromfield ES, Sinha IB, Wolynetz MS (1986) Influence of location, host cultivar and inoculation on the composition of naturalized populations of Rhizobium meliloti in Medicago sativa nodules. Appl Environ Microbiol 51:1077–1084. https://doi.org/10.1128/aem.51.5.1077-1084.1986
Bromfield ES, Wheatcroft R, Barran LR (1994) Medium for direct isolation of Rhizobium meliloti from soil. Soil Biol Biochem 26(4):423–428. https://doi.org/10.1016/0038-0717(94)90173-2
Cai J, Zhang LY, Liu W, Tian Y, Xiong JS, Wang YH, Li RJ, Li HM, Wen J, Mysore KS, Boller T, Xie ZP, Staehelin C (2018) Role of the Nod factor hydrolase MtNFH1 in regulating nod factor levels during rhizobial infection and in mature nodules of Medicago truncatula. Plant Cell 30(2):397–414. https://doi.org/10.1105/tpc.17.00420
Castonguay Y, Laberge S, Brummer EC, Volenec JJ (2006) Alfalfa winter hardiness: a research retrospective and integrated perspective. Adv Agron 90:203–265. https://doi.org/10.1016/S0065-2113(06)90006-6
Castonguay Y, Michaud R, Nadeau P, Bertrand A (2009) An indoor screening method for improvement of freezing tolerance in alfalfa. Crop Sci 49:809–818. https://doi.org/10.2135/cropsci2008.09.0539
Castonguay Y, Bertrand A, Michaud R, Laberge S (2011) Cold-induced biochemical and molecular changes in alfalfa populations selectively improved for freezing tolerance. Crop Sci 51:2132–2144. https://doi.org/10.2135/cropsci2011.02.0060
Concha C, Doerner P (2020) The impact of the rhizobia–legume symbiosis on host root system architecture. J Exp Bot 71(13):3902–3921. https://doi.org/10.1093/jxb/eraa198
Dhont C, Castonguay Y, Nadeau P, Bélanger G, Chalifour FP (2002) Alfalfa root carbohydrates and regrowth potential in response to fall harvests. Crop Sci 42(3):754–765. https://doi.org/10.2135/cropsci2002.7540
Dhont C, Castonguay Y, Nadeau P, Belanger G, Chalifour FP (2003) Alfalfa root nitrogen reserves and regrowth potential in response to fall harvests. Crop Sci 43:181–194. https://doi.org/10.2135/cropsci2003.1810
Ferguson BJ, Indrasumunar A, Hayashi S, Lin MH, Lin YH, Reid DE, Gresshoff PM (2010) Molecular analysis of legume nodule development and autoregulation. J Integr Plant Biol 52(1):61–76. https://doi.org/10.1111/j.1744-7909.2010.00899.x
Ferguson BJ, Mens C, Hastwell AH, Zhang M, Su H, Jones CH, Chu X, Gresshoff PM (2019) Legume nodulation: the host controls the party. Plant Cell Environ 42(1):41–51. https://doi.org/10.1111/pce.13348
Friel CA, Friesen ML (2019) Legumes modulate allocation to rhizobial nitrogen fixation in response to factorial light and nitrogen manipulation. Front Plant Sci 10:1316. https://doi.org/10.3389/fpls.2019.01316
Goh CH, Nicotra AB, Mathesius U (2016) The presence of nodules on legume root systems can alter phenotypic plasticity in response to internal nitrogen independent of nitrogen fixation. Plant Cell Environ 39:883–896. https://doi.org/10.1111/pce.12672
Gurusamy C, Davis PJ, Bal AK (2000) Seasonal changes in perennial nodules of beach pea (Lathyrus maritimus [L.] Bigel.) with special reference to oleosomes. Int J Plant Sci 161(4):631–638. https://doi.org/10.1086/314291
Irshad A, Rehman RNU, Kareem HA, Yang P, Hu T (2021) Addressing the challenge of cold stress resilience with the synergistic effect of Rhizobium inoculation and exogenous melatonin application in Medicago truncatula. Ecotoxicol Environ Saf 226:112816. https://doi.org/10.1016/j.ecoenv.2021.112816
Jozefkowicz C, Brambilla S, Frare R, Stritzler M, Puente M, Piccinetti C, Soto G, Ayub N (2017) Microevolution rather than large genome divergence determines the effectiveness of Legume-Rhizobia symbiotic interaction under field conditions. J Mol Evol 85(3–4):79–83. https://doi.org/10.1007/s00239-017-9808-6
Kaschuk G, Hungria M, Leffelaar PA, Giller KE, Kuyper TW (2010) Differences in photosynthetic behaviour and leaf senescence of soybean (Glycine max [L.] Merrill) dependent on N2 fixation or nitrate supply. Plant Biol 12(1):60–69. https://doi.org/10.1111/j.1438-8677.2009.00211.x
Knežević M, Berić T, Buntić A, Jovković M, Avdović M, Stanković S, Delić D, Stajković-Srbinović O (2022) Native Mesorhizobium strains improve yield and nutrient composition of the common bird’s-foot trefoil grown in an acid soil. Rhizosphere 21(100487):2452–2198. https://doi.org/10.1016/j.rhisph.2022.100487
Knight JD (2007) Evaluation of Rhizobium inoculant formulations for alfalfa yield and N fixation. Can J Plant Sci 87:267–272. https://doi.org/10.4141/P05-143
Li X, Brummer EC (2012) Applied genetics and genomics in alfalfa breeding. Agronomy 2:40–61. https://doi.org/10.3390/agronomy2010040
Li H, Testerink C, Zhang Y (2021) How roots and shoots communicate through stressful times. Trends Plant Sci 26(9):940–952. https://doi.org/10.1016/j.tplants.2021.03.005
Liu YS, Geng JC, Sha XY, Zhao YX, Hu TM, Yang PZ (2019) Effect of Rhizobium symbiosis on low-temperature tolerance and antioxidant response in alfalfa (Medicago sativa L.). Front Plant Sci 10:538. https://doi.org/10.3389/fpls.2019.00538
Marquez-Garcia B, Shaw D, Cooper JW, Karpinska B, Quain MD, Makgopa EM, Kunert K, Foyer CH (2015) Redox markers for drought-induced nodule senescence, a process occurring after drought-induced senescence of the lowest leaves in soybean (Glycine max). Ann Bot 116(4):497–510. https://doi.org/10.1093/aob/mcv030
Michaud R, Richard C, Willhmot C, Gasser HR (1983) Apica Alfalfa. Can J. Plant Sci 63(2):547–549. https://doi.org/10.4141/cjps83-066
Mueller SC, Fick GW (1989) Converting alfalfa development measurements from mean stage by count to mean stage by weight. Crop Sci 29:821–823. https://doi.org/10.2135/cropsci1989.0011183X002900030057x
Nieman T, Hoogzaad Y, Marcotte SJE, Ryser P (2018) Contrasting root overwintering strategies of perennial wetland monocots. Botany 96(10):653–661. https://doi.org/10.1139/cjb-2018-0065
Olsen P, Wright S, Collins M, Rice W (1994) Patterns of reactivity between a panel of monoclonal antibodies and forage Rhizobium strains. Appl Environ Microb 60(2):654–661. https://doi.org/10.1128/aem.60.2.654-661.1994
Parvin S, Uddin S, Tausz-Posch S, Armstrong R, Tausz M (2020) Carbon sink strength of nodules but not other organs modulates photosynthesis of faba bean (Vicia faba) grown under elevated [CO2 ] and different water supply. New Phytol 227(1):132–145. https://doi.org/10.1111/nph.16520
Pate JS (1961) Perennial nodules on native legumes in the British Isles. Nature 28:376–377
Prévost D, Drouin P, Antoun H (1999) The potential use of cold-adapted rhizobia to improve symbiotic nitrogen fixation in legumes cultivated in temperate regions. In: Margesin R, Schinner F (eds) Biotechnological Applications of Cold-Adapted Organisms. Springer, Berlin, pp 161–176
Prévost D, Drouin P, Laberge S, Bertrand A, Cloutier J, Levesque G (2003) Cold-adapted rhizobia for nitrogen fixation in temperate regions. Can J Bot 81:1153–1161. https://doi.org/10.1139/b03-113
Regus JU, Gano KA, Hollowell AC, Sofish V, Sachs JL (2015) Lotus hosts delimit the mutualism–parasitism continuum of Bradyrhizobium. J Evol Biol 28:447–456. https://doi.org/10.1111/jeb.12579
Rice W, Olsen P, Collins M (1995) Symbiotic effectiveness of Rhizobium meliloti at low root temperature. Plant Soil 170(2):351–358. https://doi.org/10.1007/BF00010489
Risula D (2019) Nodulation and nitrogen fixation field assessment guide, Saskatchewan pulse growers. 190619_Nodulation_and_Nitrogen_Fixation_Field_Assessment _Guide.pdf
Sanz-Sáez Á, Erice G, Aguirreolea J, Irigoyen JJ, Sánchez-Díaz M (2012) Alfalfa yield under elevated CO2 and temperature depends on the Sinorhizobium strain and growth season. Environ Exp Bot 77:267–273. https://doi.org/10.1016/j.envexpbot.2011.11.017
Sindhu S, Dahiya A, Gera R, Sindhu SS (2020) Mitigation of abiotic stress in legume-nodulating rhizobia for sustainable crop production. Agric Res 9:444–459. https://doi.org/10.1007/s40003-020-00474-3
Song T, Xu H, Sun N, Jiang L, Tian P, Yong Y, Yang W, Cai H, Cui G (2017) Metabolomic analysis of alfalfa (Medicago sativa L.) root-symbiotic rhizobia responses under alkali stress. Front Plant Sci 8:1208. https://doi.org/10.3389/fpls.2017.01208
Statistics Canada (2022) Alfalfa and alfalfa mixture. In: 32–10–0309–01 Field crops and hay, Census of Agriculture, 2021. Available via. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action. Accessed 20 May 2022
Staudinger C, Mehmeti-Tershani V, Gil-Quintana E, Gonzalez EM, Hofhansl F, Bachmann G, Wienkoop S (2016) Evidence for a rhizobia-induced drought stress response strategy in Medicago truncatula. J Proteom 136:202–213. https://doi.org/10.1016/j.jprot.2016.01.006
Vincent JM (1970) A Manual for the Practical Study of the Root-Nodule Bacteria. Blackwell Sci Pub, Oxford
Westhoek A, Clark LJ, Culbert M, Dalchau N, Griffiths M, Jorrin B, Karunakaran R, Ledermann R, Tkacz A, Webb I, James EK, Poole PS, Turnbull LA (2021) Conditional sanctioning in a legume-Rhizobium mutualism. Proc Natl Acad Sci 118(19):e2025760118. https://doi.org/10.1073/pnas.2025760118
Yuan K, Reuckling M, Ramirez M, Djedidi S, Fukuhara I, Ohyama T, Yokoyama T, Bellingrath-Kimura SD, Halwani M, Egamberdieva D, Ohkama-Ohtsu N (2020) Characterization of rhizobia for the improvement of soybean cultivation at cold conditions in central Europe. Microbes Environ 35(1):ME19124. https://doi.org/10.1264/jsme2.ME19124
Zhang X, Wang L, Li J, Batsone RT, Frederickson ME (2020) Medicago truncatula adjusts root proliferation, nodule formation, and partner choice in response to local N heterogeneity. Plant Soil 450:417–428. https://doi.org/10.1007/s11104-020-04433-3
Zhu J, Zhang KX, Wang WS, Gong W, Liu WC, Chen HG, Xu HH, Lu YT (2015) Low temperature inhibits root growth by reducing auxin accumulation via ARR1/12. Plant Cell Physiol 56(4):727–736. https://doi.org/10.1093/pcp/pcu217
Acknowledgements
This work was supported by a grant program of Agriculture and Agri-Food Canada. The first author was financially supported by a scholarship from the Fonds de Recherche Québécois Nature et Technologies. The authors sincerely thank Josée Bourassa, Josée Michaud and Sandra Delaney for their technical assistance. We also thank, Dr Nicolas Ayub from Instituto de Genética “Edwald Alfredo Favret”, INTA who kindly provided us the commercial S. meliloti strain, B399.
Funding
Open Access provided by Agriculture & Agri-Food Canada. This work was supported by a grant program of Agriculture and Agri-Food Canada. The first author was financially supported by scholarship from the Fonds de Recherche Québécois Nature et Technologies.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Emmanuelle D’Amours, Annick Bertrand and Jean Cloutier. The first draft of the manuscript was written by Emmanuelle D’Amours and all authors revised the previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Tim S. George.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
D’Amours, E., Bertrand, A., Cloutier, J. et al. Impact of Sinorhizobium meliloti strains and plant population on regrowth and nodule regeneration of alfalfa after a freezing event. Plant Soil (2022). https://doi.org/10.1007/s11104-022-05662-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-022-05662-4