Abstract
Background and aims
Calcium deficiency is evident in populations worldwide and combating this could be addressed with the biofortification of staple crops. Consequently, identification of Ca concentration in breeding trials would be essential. Here we report the use of energy dispersive x-ray fluorescence (EDXRF) for the rapid screening of Ca concentration in seed samples.
Methods
Grain samples of rice, wheat, pearl millet, bean, maize, and cowpea were provided from biofortification breeding programs and analysed with inductively coupled plasma – mass spectrometry (ICP-MS) to determine the reference Ca concentration. A set of samples were used to develop a Ca EDXRF calibration for each crop and another set of samples to validate the method.
Results
The EDXRF results were comparable to the reference ICP-MS analysis with an average difference of < ± 5 mg kg-1 for all crops and RSD for duplicate analysis < 10% for all crops.
Conclusions
EDXRF is suitable for the rapid analysis of Ca in bean, cowpea, wheat, pearl millet, maize and rice samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Micronutrient malnutrition results from the lack of essential micronutrients in the diet and is particularly prevalent in developing countries with a high reliance on calorie dense, but micronutrient low, staple food crops. It is estimated that approximately 2 billion people worldwide are impacted by micronutrient malnutrition (FAO 2020) and at least 50% of children worldwide suffer from micronutrient deficiencies (UNICEF 2019).
Calcium is one such essential nutrient required for various biological and physiological process in the body. Ca plays a role in signalling development of bone and teeth and has a vital structural role (99% of Ca in the body is stored in the skeleton) (Knez and Stangoulis, 2021). This mineral is also required for muscle contractions and relaxation, nerve and hormone function, and blood pressure regulation (Pravina et al. 2013). The recommended Ca dietary intake is 800 – 1,300 mg/day for adults and 1,300 mg/day for children above 9 years of age (FAO/WHO, 2002). Adequate Ca intake and retention is required throughout periods of growth, particularly during the first 2 years of life and during puberty and adolescence, and these age groups constitute populations at risk for Ca deficiency. Additionally, pregnant women (especially in the last trimester), lactating women, postmenopausal women and possibly elderly men are also at risk of Ca deficiency (FAO/WHO, 2005). Low Ca intake during pregnancy is linked to pre-eclampsia, prenatal hypertension and can negatively impact foetal development. Ca deficiency can affect skeletal growth and development and is linked to rickets in children and osteoporosis with Ca deficient older people (Frossard et al. 2000). Additionally diets with low Ca can be linked to hypertension and various cancers (Dayod et al. 2010). A study of worldwide dietary intake showed daily Ca intake can vary from 175 – 1233 mg (Balk et al. 2017). The countries with particularly low Ca intake were predominantly in Asia, Africa, and South America (Balk et al. 2017). Up to 3.5 billion people worldwide were at risk of Ca deficiency in 2011 (Kumssa et al. 2015) and Ca deficiency is almost physically undetectable and difficult to diagnose during its preliminary stages (Wang et al. 2013).
Dairy is one of the richest dietary sources of Ca, however total dietary energy intake from dairy products is quite low (~14%) in developed countries and even less (~4%) in developing countries (Silanikove et al. 2015). Additionally, approximately 65% of the world’s population is lactose-intolerant (Puranik et al. 2017) further limiting potential Ca intake from dairy. Whilst Ca deficiency is evident in developed countries, it is more prevalent in developing countries due to a lack of dietary diversity and availability of nutrient dense crops. Ca intake in developing countries is most likely from vegetables, grains and legumes (Cormick and Belizán 2019) with a high caloric reliance on staple food crops such as rice, wheat and maize. Consequently, investigations into biofortification of Ca in staple food crops is a useful approach to increase dietary Ca intake and combat micronutrient malnutrition in developing countries.
Biofortification of staple food crops to improve human nutrition has been a significant focus for many plant breeding programs and has resulted in a significant increase in Fe and/or Zn concentration in staple food crops (Saltzman et al. 2017). Despite the focus on Fe and Zn there are other essential mineral nutrients which could also be targeted with conventional plant breeding programs and have the potential to improve human nutrition. Calcium is one such essential element, increasing Ca concentration in staple food crops has the potential to improve the Ca status of some of the most vulnerable communities in the world (Knez and Stangoulis, 2021). High genotypic variation in Ca concentration and heritability has been shown in many crops (Alomari et al. 2017; Beebe et al. 2000; Gomez-Becerra et al. 2010; Sharma et al. 2017; Zhang et al. 2009) making breeding for Ca biofortified products an obvious step forward.
Conventionally, techniques such as atomic absorption spectroscopy (AAS) and inductively coupled plasma – optical emission spectroscopy (ICP-OES) or – mass spectrometry (ICP-MS) has been used for analysis of Ca concentration in plant samples (Zarcinas et al. 2008). These methods are well established and have good accuracy and precision, however such analyses require specialised equipment, high purity reagents and highly trained staff. Additionally, these analyses require samples to be ground, weighed, digested, diluted and analysed resulting in an expensive and time-consuming process. We have previously shown the benefits of using EDXRF to screen Fe and Zn concentration in breeding programs (Guild et al. 2017; Guild and Stangoulis 2021; Paltridge et al. 2012a; Paltridge et al. 2012b). EDXRF requires minimal sample preparation and a fast non-destructive analysis requiring minimal consumables and a rapid analysis time. EDXRF analysis of Ca in various food products has been reported previously (Perring and Andrey 2003; Perring and Andrey 2018; Perring et al. 2019) in plants and food crops (Ekinci et al. 2004; Guild and Stangoulis 2021; Kalcsits 2016). To develop a robust XRF calibration it is essential to have samples with robust reference values and a wide range of concentrations for the element(s) of interest.
We have previously shown it is possible to develop an EDXRF Ca calibration for whole grain sorghum (sorghum bicolor) and lentil (lens culinaris) (Guild and Stangoulis 2021) and here we present data for Ca analysis for more of the HarvestPlus crops of interest, specifically, rice (Oryza sativa L.), wheat (Triticum aestivum L.), pearl millet (Pennisetum glaucum L.), bean (Phaseolus vulgaris L.), maize (Zea mays L.) and cowpea (Vigna unguiculata L.).
Materials & methods
Samples
EDXRF calibration samples for Ca in grains are not commercially available, consequently a set of calibration and validation samples were developed for each of the crops analysed. Samples were provided from various breeding programs: beans were provided from the International Centre for Tropical Agriculture (CIAT) in Colombia, cowpea samples were provided from University of California, Riverside, USA and the International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), India, maize samples from the International Institute of Tropical Agriculture (IITA), Nigeria, wheat samples from International Maize and Wheat Improvement Centre (CIMMYT), Mexico, pearl millet samples from International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), India and rice from the International Center for Tropical Agriculture (CIAT), Colombia. All samples were sterilised with gamma irradiation at 50 kGray (5 Mrad). Flour samples were ground with a Retch Mixer Mill MM 400 with ZrO grinding jars and balls (Retsch GmbH & Co KG, Haan, Germany). Robust reference values were achieved with ICP-MS analysis at Flinders University. Approximately 0.3g of each sample was digested using a closed-tube digestion method (Knez et al. 2018; Wheal et al. 2011). Reference material for each crop was analysed for quality control for each ICP-MS analysis batch. Specifically, string bean pods WEPAL IPE192 for maize, bean, cowpea and pearl millet analysis, NIST wheat flour 1567b for wheat analysis and NIES rice flour 10-c for rice analysis.
EDXRF
A Bruker S2 Puma EDXRF fitted with a 20-position sample tray was used to acquire all EDXRF data. Acquisition parameters are summarised in Table 1 with the acquisition time and sample preparation for each crop shown in Table 2. Flour samples were prepared with a Retsch Mixer Mill MM 400 with ZrO grinding jars and balls (Retsch GmbH & Co KG, Haan, Germany), whole grain samples were analysed as received with no further preparation. As reported previously (Guild et al. 2017; Guild and Stangoulis 2021; Paltridge et al. 2012a; Paltridge et al. 2012b), analysis was performed on samples of >5g in supplied 35 mm diameter sample cups sealed with 4 μm Poly-4 (polypropylene) XRF film.
Statistics
Calibration and validation statistics reported are defined below (Perring and Andrey 2003)
Concentration determined by ICP-MS | \({y}_{i}\) |
Concentration determined by EDXRF | \(\widehat{{y}_{i}}\) |
Bias | \(\frac{{\sum }_{i=1}^{n}\left(\widehat{{y}_{i}}- {y}_{i}\right)}{n}\) |
Standard error of prediction (SEP) | \(\sqrt{\frac{{\sum }_{i=1}^{n}{\left(\widehat{{y}_{i}}-{y}_{i}\right)}^{2}}{n}}\) |
Standard error of calibration (SEC) | \(\sqrt{\frac{{\sum }_{i=1}^{n}{\left(\widehat{{y}_{i}}-{y}_{i}\right)}^{2}}{n-p-1}}\) |
Limit of detection (LOD) as calculated by the Bruker EDXRF calibration software with limit of quantification (LOQ) determined as 10/3 LOD.
Results
Calibration
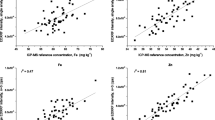
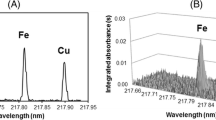
Ca EDXRF calibration was developed for each crop analysed with all showing a strong correlation between EDXRF intensity and the reference ICP-MS concentration (Figure 1). Ca calibrations for each of the crops were developed for the Ca Kα fluorescence peak (corrected for K Kβ overlap) and the resulting calibration statistics are shown in Table 3. These results show strong calibrations (r2 ≥ 0.86) for all the crops analysed and standard error of calibration (SEC) ranged from ± 7.43 – 75.39 mg kg-1 with LOQ ranging from 18 – 202 mg kg-1.
Validation
The validation statistics for the Ca calibrations for each crop all showed r2 ≥ 0.93 (Table 4 and Figure 2). The average bias between reference ICP-MS and EDXRF analysis was less than ± 5 mg kg-1 for all crops and not significantly different from zero (at the 95% level, according to paired t-test). The 95% confidence interval was < 20 mg kg-1 for bean and cowpea and < 8 mg kg-1 for all other crops analysed. EDXRF reproducibility for duplicate analysis (RSD) was less than 10 % for all crops analysed.
Discussion
The benefit of EDXRF for elemental analysis is the rapid throughput and minimal sample preparation. We have previously shown the suitability of this method for screening Fe and Zn concentration in each of the crops analysed here, specifically for wheat (Paltridge et al. 2012a), rice and pearl millet (Paltridge et al. 2012b) and bean, maize and cowpea (Guild et al. 2017). Increasing Fe and Zn has been the focus of many biofortification programs and has had great success for improving human nutrition. However, there is potential to expand breeding programs in the future to study more elements of nutritional importance, such as Ca. Consequently, developing a rapid screening technique for Ca is essential.
Ca is a relatively light element, consequently Ca fluorescence is more significantly attenuated in the air atmosphere (Towett et al. 2016) when compared to heavier elements (e.g., Fe and Zn). To mitigate this effect, Ca analysis with XRF is often conducted under vacuum (Perring and Andrey 2018; Perring and Blanc 2008; Perring et al. 2019; Perring et al. 2016). However, the requirement for samples being ground, pressed and analysed under a vacuum increases both the time and cost of analysis. It has previously been shown that Ca analysis of whole seeds under an air atmosphere is feasible (Guild and Stangoulis 2021). These results show it is possible to analyse the smaller seed crops (rice and pearl millet) as whole grain and still produce a robust calibration and validation (r2 > 0.9), however the remaining crops required seed to be ground to flour to produce suitably robust calibrations as seen in Figure 1. The crops studied here span a large range of Ca concentrations, from 150 to 200 mg kg-1 in rice and maize to approximately 1000 to 2000 mg kg-1 in beans. To achieve reproducible and accurate Ca results, the analysis time was optimised for each crop. Calibration data was acquired at successively longer acquisition times (30 s, 60 s, 120 s, 240 s) to produce a correlation between EDXRF intensity and ICP-MS reference concentration of r2 > 0.85. It is evident that crops with lower Ca concentrations and/or Ca range require longer analysis time (Table 2) to produce suitably robust calibrations (Table 3). Ca calibrations for bean and cowpea (crops with comparably high levels of Ca) were achieved with 30s scan time. This is the same as the acquisition parameters for analysis of Fe and Zn (Guild et al. 2017) and would require no changes to the current protocols in order to simultaneously achieve Ca screening (as shown in Figure 3). However, crops with lower concentrations of Ca (wheat, pearl millet, maize and rice) require longer scanning times to achieve suitably robust Ca analysis for breeding programs. Whilst increasing the analysis time would have the added benefit of improving the accuracy of Fe and Zn EDXRF analysis, this could significantly impact sample throughput if all samples from breeding trials were scanned at this longer time. Consequently, it may be desirable for large breeding programs to analyse only a subset of samples with this longer analysis time rather than scanning all samples for 240s. However, in comparison to conventional ICP analysis, which requires significant sample preparation, digestion, dilution, and analysis steps along with samples generally requiring domestic or even international shipment to a dedicated off-site laboratory, an analysis of 240s is still an improvement. The reproducibility of XRF is < 10%, while this is higher than that observed with ICP, this is still more than suitable for high throughput screening. The only consumable required for XRF analysis is the preparation of sample cups at a cost of ~$US 0.15 per sample. This combined with minimal sample preparation and labour time results in significant reductions in analysis cost per sample when comparing XRF and ICP (<$1 and >$25 respectively) and the ability to analyse samples at the field station, all indicate XRF is suitable for rapid screening for Ca in breeding programs.
Conclusions
Rapid screening for micronutrient concentration in biofortification breeding programs is essential. The fast and simple analysis achieved with EDXRF is the ideal tool for this and is well established for screening Fe and Zn in multiple crops. Here we have confirmed this technique can also be used for screening Ca in many staple food crops. The results indicate a strong correlation between EDXRF and the reference ICP-MS analysis for all crops, with r2 > 0.93. Whilst longer analysis time is required than is used for Fe and Zn, this Ca method is still significantly faster and easier than the conventional wet analysis techniques. Additionally, this non-destructive technique is much cheaper at a cost of < $US 0.15 per sample. These results have shown (with 95% confidence) the EDXRF results are comparable with ICP-MS with results < ± 2.5 mg kg-1 for rice and maize, <± 8 mg kg-1 for wheat and pearl millet at < ± 20 mg kg-1 for bean and cowpea. These results provide an essential screening platform for plant breeders aiming to develop Ca-enriched staple crops.
Availability of data and material
Not applicable
References
Alomari DZ, Eggert K, von Wiren N, Pillen K, Roder MS (2017) Genome-Wide Association Study of Calcium Accumulation in Grains of European Wheat Cultivars. Front Plant Sci 8:1797. https://doi.org/10.3389/fpls.2017.01797
Balk EM, Adam GP, Langberg VN, Earley A, Clark P, Ebeling PR, Mithal A, Rizzoli R, Zerbini CAF, Pierroz DD, Dawson-Hughes B (2017) Global dietary calcium intake among adults: a systematic review. Osteoporosis international 28:3315–3324. https://doi.org/10.1007/s00198-017-4230-x
Beebe S, Gonzalez AV, Rengifo J (2000) Research on Trace Minerals in the Common Bean. Food and Nutrition Bulletin 21:387–391. https://doi.org/10.1177/156482650002100408
Cormick G, Belizán JM (2019) Calcium Intake and Health. Nutrients 11:1606. https://doi.org/10.3390/nu11071606
Dayod M, Tyerman SD, Leigh RA, Gilliham M (2010) Calcium storage in plants and the implications for calcium biofortification. Protoplasma 247:215–231. https://doi.org/10.1007/s00709-010-0182-0
Ekinci N, Ekinci R, Polat R, Budak G (2004) Analysis of trace elements in medicinal plants with energy dispersive X-ray fluorescence. J Radioanal Nucl Ch 260:127–131. https://doi.org/10.1023/B:JRNC.0000027071.72742.ee
FAO, WHO, (2002) Report of a joint FAO/WHO expert consultation on human vitamin and mineral requirements. FAO, Rome
FAO/ WHO (2005) Vitamin and mineral requirements in human nutrition. World Health Organization.
Frossard E, Bucher M, Machler F, Mozafar A, Hurrell R (2000) Potential for increasing the content and bioavailability of Fe, Zn and Ca in plants for human nutrition. Journal of the Science of Food and Agriculture 80:861–879. https://doi.org/10.1002/(Sici)1097-0010(20000515)80:7%3c861::Aid-Jsfa601%3e3.3.Co;2-G
Gomez-Becerra HF, Erdem H, Yazici A, Tutus Y, Torun B, Ozturk L, Cakmak I (2010) Grain concentrations of protein and mineral nutrients in a large collection of spelt wheat grown under different environments. Journal of Cereal Science 52:342–349. https://doi.org/10.1016/j.jcs.2010.05.003
Guild GE, Paltridge NG, Andersson MS, Stangoulis JCR (2017) An energy-dispersive X-ray fluorescence method for analyzing Fe and Zn in common bean, maize and cowpea biofortification programs. Plant Soil 419:457–466. https://doi.org/10.1007/s11104-017-3352-4
Guild GE, Stangoulis JCR (2021) EDXRF for screening micronutrients in lentil and sorghum biofortification breeding programs. Plant and Soil. https://doi.org/10.1007/s11104-021-04922-z
Kalcsits LA (2016) Non-destructive Measurement of Calcium and Potassium in Apple and Pear Using Handheld X-ray Fluorescence. Front Plant Sci 7:442. https://doi.org/10.3389/fpls.2016.00442
Knez M, Tako E, Glahn RP, Kolba N, de Courcy-Ireland E, Stangoulis JCR (2018) Linoleic Acid:Dihomo-gamma-Linolenic Acid Ratio Predicts the Efficacy of Zn-Biofortified Wheat in Chicken (Gallus gallus). J Agric Food Chem 66:1394–1400. https://doi.org/10.1021/acs.jafc.7b04905
Knez M, Stangoulis JCR (2021) Calcium Biofortification of Crops-Challenges and Projected Benefits. Frontiers in Plant Science 12. https://doi.org/10.3389/fpls.2021.669053
Kumssa DB, Joy EJM, Ander EL, Watts MJ, Young SD, Walker S, Broadley MR (2015) Dietary calcium and zinc deficiency risks are decreasing but remain prevalent. Scientific Reports 5:10974. https://doi.org/10.1038/srep10974
Paltridge NG, Milham PJ, Ortiz-Monasterio JI, Velu G, Yasmin Z, Palmer LJ, Guild GE, Stangoulis JCR (2012) Energy-dispersive X-ray fluorescence spectrometry as a tool for zinc, iron and selenium analysis in whole grain wheat. Plant and Soil 361:261–269. https://doi.org/10.1007/s11104-012-1423-0
Paltridge NG, Palmer LJ, Milham PJ, Guild GE, Stangoulis JCR (2012) Energy-dispersive X-ray fluorescence analysis of zinc and iron concentration in rice and pearl millet grain. Plant and Soil 361:251–260. https://doi.org/10.1007/s11104-011-1104-4
Perring L, Andrey D (2003) ED-XRF as a tool for rapid minerals control in milk-based products. J Agric Food Chem 51:4207–4212. https://doi.org/10.1021/jf034158p
Perring L, Andrey D (2018) Multi-elemental ED-XRF Determination in Dehydrated Bouillon and Sauce Base Products. Food Anal Method 11:148–160. https://doi.org/10.1007/s12161-017-0985-0
Perring L, Blanc J (2008) Validation of quick measurement of mineral nutrients in milk powders: comparison of energy dispersive X-ray fluorescence with inductively coupled plasma-optical emission spectroscopy and potentiometry reference methods. Sensing and Instrumentation for Food Quality and Safety 2:254–261. https://doi.org/10.1007/s11694-008-9056-y
Perring L, Cotard A, Al Sayadi S, Berrut S (2019) Rapid analysis of Na, Mg, Ca, Fe, and Zn in breakfast cereals (granola type) by energy dispersive-X-ray fluorescence. X-Ray Spectrom 48:395–400. https://doi.org/10.1002/xrs.2988
Perring L, Nicolas M, Andrey D, Fragnière Rime C, Richoz-Payot J, Dubascoux S, Poitevin E (2016) Development and Validation of an ED-XRF Method for the Fast Quantification of Mineral Elements in Dry Pet Food Samples. Food Anal Method 10:1469–1478. https://doi.org/10.1007/s12161-016-0695-z
Pravina P, Sayaji D, Avinash M (2013) Calcium and its Role in Human Body. International Journal of Research in Pharmaceutical and Biomedical Sciences 4:659–668
Puranik S, Kam J, Sahu PP, Yadav R, Srivastava RK, Ojulong H, Yadav R (2017) Harnessing Finger Millet to Combat Calcium Deficiency in Humans: Challenges and Prospects. Front Plant Sci 8:1311. https://doi.org/10.3389/fpls.2017.01311
Saltzman A, Birol E, Oparinde A, Andersson MS, Asare-Marfo D, Diressie MT, Gonzalez C, Lividini K, Moursi M, Zeller M (2017) Availability, production, and consumption of crops biofortified by plant breeding: current evidence and future potential. Ann N Y Acad Sci 1390:104–114. https://doi.org/10.1111/nyas.13314
Sharma D, Jamra G, Singh UM, Sood S, Kumar A (2017) Calcium Biofortification: Three Pronged Molecular Approaches for Dissecting Complex Trait of Calcium Nutrition in Finger Millet (Eleusine coracana) for Devising Strategies of Enrichment of Food Crops. Frontiers in plant science 7:2028. https://doi.org/10.3389/fpls.2016.02028
Silanikove N, Leitner G, Merin U (2015) The Interrelationships between Lactose Intolerance and the Modern Dairy Industry: Global Perspectives in Evolutional and Historical Backgrounds. Nutrients 7:7312–7331. https://doi.org/10.3390/nu7095340
Towett EK, Shepherd KD, Drake BL (2016) Plant elemental composition and portable X-ray fluorescence (pXRF) spectroscopy: quantification under different analytical parameters. X-Ray Spectrom 45:117–124. https://doi.org/10.1002/xrs.2678
UNICEF (2019) The state of the world’s children 2019: children, food and nutrition: growing well in a changing world. UNICEF, New York
Wang M, Yang X, Wang F, Li R, Ning H, Na L, Huang Y, Song Y, Liu L, Pan H, Zhang Q, Fan L, Li Y, Sun C (2013) Calcium-deficiency assessment and biomarker identification by an integrated urinary metabonomics analysis. BMC Medicine 11:86. https://doi.org/10.1186/1741-7015-11-86
Wheal MS, Fowles TO, Palmer LT (2011) A cost-effective acid digestion method using closed polypropylene tubes for inductively coupled plasma optical emission spectrometry (ICP-OES) analysis of plant essential elements. Analytical Methods 3:2854–2863. https://doi.org/10.1039/c1ay05430a
FAO, IFAD, UNICEF, WFP and WHO. (2020). The State of Food Security and Nutrition in the World 2020. Transforming food systems for affordable healthy diets. Rome, FAO
Zarcinas BA, Cartwright B, Spouncer LR (2008) Nitric acid digestion and multi-element analysis of plant material by inductively coupled plasma spectrometry. Communications in Soil Science and Plant Analysis 18:131–146. https://doi.org/10.1080/00103628709367806
Zhang B, Chen P, Shi A, Hou A, Ishibashi T, Wang D (2009) Putative Quantitative Trait Loci Associated with Calcium Content in Soybean Seed. Journal of Heredity 100:263–269. https://doi.org/10.1093/jhered/esn096
Acknowledgements
This study was made possible with support from HarvestPlus. HarvestPlus’ principal donors are the UK Government; the Bill & Melinda Gates Foundation; the US Government’s Feed the Future initiative; Global Affair Canada; the European Commission; the Children’s Investment Fund Foundation, and donors to the CGIAR Research Program on Agriculture for Nutrition and Health (A4NH). HarvestPlus is also supported by the John D. and Catherine T. MacArthur Foundation. We would like to thank our collaborators at CIAT, CIMMYT, ICRISAT, IITA and University of California, Riverside for kindly providing the seeds.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This research was funded by HarvestPlus
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
All authors consent to publication of this manuscript
Code availability
Not applicable
Additional information
Responsible editor: Peter J. Gregory
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guild, G.E., Stangoulis, J.C.R. Screening Ca concentration in staple food crops with energy dispersive x-ray fluorescence (EDXRF). Plant Soil 473, 659–667 (2022). https://doi.org/10.1007/s11104-021-05283-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-021-05283-3