Abstract
Aims
Legacy effects arising from grazing may alter the effect of plant-soil feedback (PSF), plant-phyllosphere feedback (PPF) and/or transgenerational plasticity on plant performance. Understanding how effects from these mechanisms are influenced by grazing can help predict grassland production and ecosystem dynamics.
Methods
Plant clonal buds and shoot materials of Leymus chinensis (a clonal grass species widely distributed across the eastern Eurasian Steppes), sterilised and non-sterilised soils were collected from sites subjected to long-term grazing or no grazing. We then conducted a series of studies to determine the presence and context-dependency of (1) PSF, (2) PPF and (3) clonal transgenerational plasticity mechanisms on long-term grazing by measuring plant above/belowground biomass, height and density.
Results
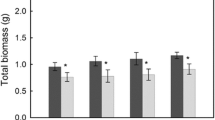
Inoculation with soil biota conditioned by L. chinensis from grazed sites increased root growth by 83.99% (positive PSF), but not inoculum from ungrazed sites (neutral PSF). Shoot inocula from grazed and ungrazed sites had similar positive effects on plant growth (positive PPF). Clonal offspring from grazed sites showed transgenerational trait plasticity in terms of herbivory-avoidance (21.24% height reduction and 84.62% tiller density increase), but aboveground production was unaffected. Additionally, grazed field plots with a year of grazing exclusion showed similar plant heights and aboveground biomass as ungrazed plots, which indicated short-term transgenerational trait plasticity.
Conclusions
Positive PSF and short-term clonal transgenerational plasticity of L. chinensis depended on past grazing activity, and together with positive PPF, can aid their recovery and fitness in subsequent growing seasons. Our findings highlighted context-dependent plant–microorganism interactions and trans-generational feedback of plants in response to grazing.




Similar content being viewed by others
References
Abril AB, Torres PA, Bucher EH (2005) The importance of phyllosphere microbial populations in nitrogen cycling in the Chaco semi-arid woodland. J Trop Ecol: 103–107.
Agrawal AA (2001) Transgenerational consequences of plant responses to herbivory: an adaptive maternal effect? Am Nat 157:555–569
Bardgett RD, Wardle DA (2003) Herbivore-mediated linkages between aboveground and belowground communities. Ecology 84:2258–2268
Bardgett RD, Wardle DA (2010) Aboveground-belowground linkages: biotic interactions, ecosystem processes, and global change. Oxford University Press
Baxendale C, Orwin KH, Poly F, Pommier T, Bardgett RD (2014) Are plant–soil feedback responses explained by plant traits? New Phytol 204:408–423
Benson EJ, Hartnett DC (2006) The role of seed and vegetative reproduction in plant recruitment and demography in tallgrass prairie. Plant Ecol 187:163–178
Bezemer TM, van der Putten WH, Martens H, van de Voorde TF, Mulder PP, Kostenko O (2013) Above-and below-ground herbivory effects on below-ground plant–fungus interactions and plant–soil feedback responses. J Ecol 101:325–333
Bommarco R, Kleijn D, Potts SG (2013) Ecological intensification: harnessing ecosystem services for food security. Trends Ecol Evol 28:230–238
Brinkman E, Van der Putten WH, Bakker EJ, Verhoeven KJ (2010) Plant–soil feedback: experimental approaches, statistical analyses and ecological interpretations. J Ecol 98:1063–1073
Briske DD, Joyce LA, Polley HW, Brown JR, Wolter K, Morgan JA, McCarl BA, Bailey DW (2015) Climate-change adaptation on rangelands: linking regional exposure with diverse adaptive capacity. Front Ecol Environ 13:249–256
Carvalho SD, Castillo JA (2018) Influence of light on plant–phyllosphere interaction. Front Plant Sci 9:1482
Chen T, Christensen M, Nan Z, Hou F (2017) The effects of different intensities of long-term grazing on the direction and strength of plant–soil feedback in a semiarid grassland of Northwest China. Plant Soil 413:303–317
Chen T, Nan Z, Kardol P, Duan T, Song H, Wang J, Li C, Hou F (2018) Effects of interspecific competition on plant-soil feedbacks generated by long-term grazing. Soil Biol Biochem 126:133–143
Crawford KM, Bauer JT, Comita LS, Eppinga MB, Johnson DJ, Mangan SA, Queenborough SA, Strand AE, Suding KN, Umbanhowar J (2019) When and where plant-soil feedback may promote plant coexistence: a meta-analysis. Ecol Lett 22:1274–1284
De Deyn GB, Quirk H, Oakley S, Ostle NJ, Bardgett RD (2012) Increased plant carbon translocation linked to overyielding in grassland species mixtures. PLoS One 7:e45926
Donelson JM, Salinas S, Munday PL, Shama LN (2018) Transgenerational plasticity and climate change experiments: Where do we go from here? Global Change Biol 24:13–34
Dudenhöffer JH, Ebeling A, Klein AM, Wagg C (2018) Beyond biomass: Soil feedbacks are transient over plant life stages and alter fitness. J Ecol 106:230–241
Fang K, Chen L, Zhou J, Yang Z-P, Dong X-F, Zhang H-B (2019) Plant–soil–foliage feedbacks on seed germination and seedling growth of the invasive plant Ageratina adenophora. Proc R Soc B 286:20191520
Farré-Armengol G, Filella I, Llusia J, Peñuelas J (2016) Bidirectional interaction between phyllospheric microbiotas and plant volatile emissions. Trends Plant Sci 21:854–860
Gao Z-j, Liu J-b, An Q-q, Zhi W, Chen S-l, Xu B-c (2017) Photosynthetic performance of switchgrass and its relation to field productivity: A three-year experimental appraisal in semiarid Loess Plateau. J Integr Agric 16:1227–1235
Gibson DJ (2009) Grasses and grassland ecology. Oxford University Press
Gong T, Xin XF (2021) Phyllosphere microbiota: Community dynamics and its interaction with plant hosts. J Integr Plant Biol 63:297–304
Grant RC, Botha J, Grant TC, Peel MJ, Smit IP (2019) When less is more: heterogeneity in grass patch height supports herbivores in counter-intuitive ways. African Journal of Range & Forage Science 36:1–8
Kaisermann A, de Vries FT, Griffiths RI, Bardgett RD (2017) Legacy effects of drought on plant–soil feedbacks and plant–plant interactions. New Phytol 215:1413–1424
Kéry M, Matthies D, Spillmann HH (2000) Reduced fecundity and offspring performance in small populations of the declining grassland plants Primula veris and Gentiana lutea. J Ecol 88:17–30
Kos M, Veendrick J, Bezemer TM (2013) Local variation in conspecific plant density influences plant–soil feedback in a natural grassland. Basic Appl Ecol 14:506–514
Laforest-Lapointe I, Messier C, Kembel SW (2016) Host species identity, site and time drive temperate tree phyllosphere bacterial community structure. Microbiome 4:1–10
Laforest-Lapointe I, Messier C, Kembel SW (2017) Tree leaf bacterial community structure and diversity differ along a gradient of urban intensity. MSystems 2.
Landgraf R, Schaarschmidt S, Hause B (2012) Repeated leaf wounding alters the colonization of Medicago truncatula roots by beneficial and pathogenic microorganisms. Plant, Cell Environ 35:1344–1357
Leopold A (1949) The control of tillering in grasses by auxin. Am J Bot: 437–440.
Li X, Wu Z, Liu Z, Hou X, Badgery W, Guo H, Zhao Q, Hu N, Duan J, Ren W (2015) Contrasting effects of long-term grazing and clipping on plant morphological plasticity: evidence from a rhizomatous grass. PLoS One 10:e0141055
Li X, Hu N, Yin J, Ren W, Fry E (2021) Historic grazing enhances root-foraging plasticity rather than nitrogen absorbability in clonal offspring of Leymus chinensis. Plant Soil 466:65–79
Liu J, Wang L, Wang D, Bonser SP, Sun F, Zhou Y, Gao Y, Teng X (2012) Plants can benefit from herbivory: stimulatory effects of sheep saliva on growth of Leymus chinensis. PloS one 7:e29259
Liu Y, Ma G, Zan Z, Chen A, Miao Y, Wang D, Miao R (2018) Effects of nitrogen addition and mowing on rodent damage in an Inner Mongolian steppe. Ecol Evol 8:3919–3926
Liu H, Brettell LE, Qiu Z, Singh BK (2020) Microbiome-mediated stress resistance in plants. Trends Plant Sci 25(8):733–743
Liu M, Mipam TD, Wang X, Zhang P, Lin Z, Liu X (2021a) Contrasting effects of mammal grazing on foliar fungal diseases: patterns and potential mechanisms. New Phytol 232:345–355. https://doi.org/10.1111/nph.17324
Liu Y, Zhao C, Guo J, Zhang L, Xuan J, Chen A, You C (2021) Short-term phosphorus addition augments the effects of nitrogen addition on soil respiration in a typical steppe. Sci Total Environ 761:143211
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63:541–556
Manrubia M, van der Putten WH, Weser C, Veen C (2020) Rhizosphere and litter feedbacks to range-expanding plant species and related natives. Plant Soil 108:353–365. https://doi.org/10.1111/1365-2745.13299
Mariotte P, Mehrabi Z, Bezemer TM, De Deyn GB, Kulmatiski A, Drigo B, Veen GC, Van der Heijden MG, Kardol P (2018) Plant–soil feedback: bridging natural and agricultural sciences. Trends Ecol Evol 33:129–142
Park YS, Ryu CM (2021) Understanding plant social networking system: Avoiding deleterious microbiota but calling beneficials. Int J Mol Sci 22(7):3319
Perreault R, Laforest-Lapointe I (2021) Plant-microbe interactions in the phyllosphere: facing challenges of the anthropocene. ISME J: 1–7.
Png GK, Lambers H, Kardol P, Turner BL, Wardle DA, Laliberté E (2019) Biotic and abiotic plant–soil feedback depends on nitrogen-acquisition strategy and shifts during long-term ecosystem development. J Ecol 107:142–153
Ren W, Hu N, Hou X, Zhang J, Guo H, Liu Z, Kong L, Wu Z, Wang H, Li X (2017) Long-term overgrazing-induced memory decreases photosynthesis of clonal offspring in a perennial grassland plant. Front Plant Sci 8:419
Romero FM, Marina M, Pieckenstain FL (2016) Novel components of leaf bacterial communities of field-grown tomato plants and their potential for plant growth promotion and biocontrol of tomato diseases. Res Microbiol 167:222–233
Rosado BH, Almeida LC, Alves LF, Lambais MR, Oliveira RS (2018) The importance of phyllosphere on plant functional ecology: a phyllo trait manifesto. New Phytol 219:1145–1149
Shea N, Pen I, Uller T (2011) Three epigenetic information channels and their different roles in evolution. J Evol Biol 24:1178–1187
Stone BW, Weingarten EA, Jackson CR (2018) The role of the phyllosphere microbiome in plant health and function. Annual Plant Reviews online: 533–556.
Sun Y-L, Hong S-K (2012) Agrobacterium tumefaciens-mediated transformation of the halophyte Leymus chinensis (Trin.). Plant Mol Biol Report 30:1253–1263
Van der Putten WH, Bardgett RD, Bever JD, Bezemer TM, Casper BB, Fukami T, Kardol P, Klironomos JN, Kulmatiski A, Schweitzer JA (2013) Plant–soil feedbacks: the past, the present and future challenges. J Ecol 101:265–276
van der Putten WH, Bradford MA, Pernilla Brinkman E, van de Voorde TF, Veen G (2016) Where, when and how plant–soil feedback matters in a changing world. Funct Ecol 30:1109–1121
van Ruijven J, Ampt E, Francioli D, Mommer L (2020) Do soil-borne fungal pathogens mediate plant diversity–productivity relationships? Evidence and future opportunities. J Ecol 108(5):1810–1821
Veen G, de Vries S, Bakker ES, van der Putten WH, Olff H (2014) Grazing-induced changes in plant–soil feedback alter plant biomass allocation. Oikos 123:800–806
Vorholt JA (2012) Microbial life in the phyllosphere. Nat Rev Microbiol 10:828–840
Wang D, Du J, Zhang B, Ba L, Hodgkinson KC (2017a) Grazing intensity and phenotypic plasticity in the clonal grass Leymus chinensis. Rangeland Ecol Manage 70:740–747
Wang Z, Deng X, Song W, Li Z, Chen J (2017b) What is the main cause of grassland degradation? A case study of grassland ecosystem service in the middle-south Inner Mongolia. CATENA 150:100–107
Whipps J, Hand P, Pink D, Bending GD (2008) Phyllosphere microbiology with special reference to diversity and plant genotype. J Appl Microbiol 105(6):1744–1755
Whitaker BK, Bauer JT, Bever JD, Clay K (2017) Negative plant-phyllosphere feedbacks in native Asteraceae hosts–a novel extension of the plant-soil feedback framework. Ecol Lett 20:1064–1073
Wu J, Zhang Q, Li A, Liang C (2015) Historical landscape dynamics of Inner Mongolia: patterns, drivers, and impacts. Landscape Ecol 30:1579–1598
Wubs EJ, Bezemer TM (2016) Effects of spatial plant–soil feedback heterogeneity on plant performance in monocultures. J Ecol 104(2):364–376
Yuan J, Li H, Yang Y (2020) The compensatory tillering in the forage grass Hordeum brevisubulatum after simulated grazing of different severity. Frontiers in Plant Science 11
Zhu F, Heinen R, van der Sluijs M, Raaijmakers C, Biere A, Bezemer TM (2018) Species-specific plant–soil feedbacks alter herbivore-induced gene expression and defense chemistry in Plantago lanceolata. Oecologia 188:801–811
Funding
Funding was supported by the Natural Science Foundation (NSF) of China (32071882; 31702161), Inner Mongolia Science and Technology Project (2021GG0055; 2021ZY0039; 2021GG0415), the Open Project Program of the Ministry of Education Key Laboratory of Ecology and Resource Use of the Mongolian Plateau (Inner Mongolia University); Qinghai Science & Technology Project (2020-ZJ-Y03). We thank Qingshan Zhao, Junjie Duan, Zhuo Bai and Fang Mu for their assistance in the experiments.
Author information
Authors and Affiliations
Contributions
X.L. K.J. and Y.L. conceived the ideas and designed methodology; X.L., S.S. and H.S. collected the data; X.L. K.P., K.J. and Y.L. analyzed the data; X.L., K.P., K.J. and Y.L. led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Hans Lambers.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, X., Png, G.K., Sun, S. et al. Positive microbial legacy and short-term clonal plasticity aid grazing tolerance of a widespread grass species. Plant Soil 473, 291–303 (2022). https://doi.org/10.1007/s11104-021-05281-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-021-05281-5