Abstract
Aims
The rhizosphere and root detritusphere are hotspots of microbial activity, where root-derived inputs induce intensive priming effects (PE) on soil organic carbon (SOC) decomposition. These conditions for induced PE differ between rhizosphere and detritusphere and are modified by plant traits.
Methods
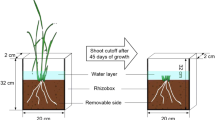
Continuous labelling with 13C-depleted CO2 allowed for the partitioning of plant and soil C sources of CO2 efflux and the investigation of the PE in the rhizosphere and detritusphere of slow-growing conservative Carex acuta and fast-growing acquisitive Glyceria maxima.
Results
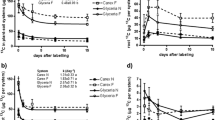
Glyceria allocated more C into the soil, induced higher microbial activity and a larger portion of active microorganisms, and depleted mineral N stronger than Carex. Its rhizosphere PE was 2.5 times stronger than that of Carex. Root residues (detritusphere) induced negative PE at the early stage of decomposition (1–9 months). The depletion of available organic substances in the detritusphere of more easily decomposable Glyceria roots resulted in positive PE after 3 months. The PE in the detritusphere of N-poorer Carex roots was more intensive but started after 9 months.
Conclusions
The rhizosphere PE was positive and stronger than the detritusphere PE, which switched from initially negative to positive PE after depletion of available substances during few months. More productive species with faster N-uptake and higher belowground C input (here Glyceria) induce larger rhizosphere PE than slower-growing species (here Carex). The N-rich Glyceria roots decompose faster than N-poor roots of Carex and, consequently, have a lower impact on SOC dynamics and induced a smaller positive detritusphere PE.
Graphic abstract





Similar content being viewed by others
Data availability
All data are included in the manuscript and supplementary materials.
Code availability
Not applicable.
References
Baggs EM (2006) Partitioning the components of soil respiration: A research challenge. Plant Soil 284:1–5
Baptist F, Aranjuelo I, Legay N, Lopez-Sangil L, Molero G, Rovira P, Nogues S (2015) Rhizodeposition of organic carbon by plants with contrasting traits for resource acquisition: Responses to different fertility regimes. Plant Soil 394:391–406
Bardgett RD, Mommer L, De Vries FT (2014) Going underground: Root traits as drivers of ecosystem processes. Trends Ecol Evol 29:692–699
Barta J, Melichova T, Vanek D, Picek T, Santruckova H (2010) Effect of ph and dissolved organic matter on the abundance of nirk and nirs denitrifiers in spruce forest soil. Biogeochemistry 101:123–132
Barta J, Slajsova P, Tahovska K, Picek T, Santruckova H (2014) Different temperature sensitivity and kinetics of soil enzymes indicate seasonal shifts in c, n and p nutrient stoichiometry in acid forest soil. Biogeochemistry 117:525–537
Bastian F, Bouziri L, Nicolardot B, Ranjard L (2009) Impact of wheat straw decomposition on successional patterns of soil microbial community structure. Soil Biol Biochem 41:262–275
Blagodatskaya E, Kuzyakov Y (2008) Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: Critical review. Biol Fertil Soils 45:115–131
Boddy E, Hill PW, Farrar J, Jones DL (2007) Fast turnover of low molecular weight components of the dissolved organic carbon pool of temperate grassland field soils. Soil Biol Biochem 39:827–835
Bruulsema TW, Duxbury JM (1996) Simultaneous measurement of soil microbial nitrogen, carbon, and carbon isotope ratio. Soil Sci Soc Am J 60:1787–1791
Butler JL, Williams MA, Bottomley PJ, Myrold DD (2003) Microbial community dynamics associated with rhizosphere carbon flow. Appl Environ Microbiol 69:6793–6800
Cardenas J, Santa F, Kaštovská E (2021) The exudation of surplus products links plant functional traits and plant-microbial stoichiometry. Land 10:840
Chen R, Senbayram M, Blagodatsky S, Myachina O, Dittert K, Lin X, Blagodatskaya E, Kuzyakov Y (2014) Soil c and n availability determine the priming effect: Microbial n mining and stoichiometric decomposition theories. Glob Change Biol 20:2356–2367
Cheng WX, Parton WJ, Gonzalez-Meler MA, Phillips R, Asao S, McNickle GG, Brzostek E, Jastrow JD (2014) Synthesis and modeling perspectives of rhizosphere priming. New Phytol 201:31–44
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Perez-Harguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, van Bodegom P, Brovkin V, Chatain A, Callaghan TV, Diaz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Victoria Vaieretti M, Westoby M (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071
Craine JM, Lee WG, Bond WJ, Williams RJ, Johnson LC (2005) Environmental constraints on a global relationship among leaf and root traits of grasses. Ecology 86:12–19
Craine JM, Morrow C, Fierer N (2007) Microbial nitrogen limitation increases decomposition. Ecology 88:2105–2113
Cros C, Alvarez G, Keuper F, Fontaine S (2019) A new experimental platform connecting the rhizosphere priming effect with co2 fluxes of plant-soil systems. Soil Biol Biochem 130:12–22
De Deyn GB, Cornelissen JHC, Bardgett RD (2008) Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol Lett 11:516–531
De Deyn GB, Quirk H, Yi Z, Oakley S, Ostle NJ, Bardgett RD (2009) Vegetation composition promotes carbon and nitrogen storage in model grassland communities of contrasting soil fertility. J Ecol 97:864–875
de Vries FT, Bardgett RD (2012) Plant-microbial linkages and ecosystem nitrogen retention: Lessons for sustainable agriculture. Front Ecol Environ 10:425–432
Dijkstra FA, Cheng W, Johnson DW (2006) Plant biomass influences rhizosphere priming effects on soil organic matter decomposition in two differently managed soils. Soil Biol Biochem 38:2519–2526
Dijkstra FA, Carrillo Y, Pendall E, Morgan JA (2013) Rhizosphere priming: A nutrient perspective. Front Microbiol 4:216
Drake JE, Darby BA, Giasson MA, Kramer MA, Phillips RP, Finzi AC (2013) Stoichiometry constrains microbial response to root exudation-insights from a model and a field experiment in a temperate forest. Biogeosciences 10:821–838
Evans JR (1989) Photosynthesis and nitrogen relationships in leaves of c3 plants. Oecologia 78:9–19
Finzi AC, Abramoff RZ, Spiller KS, Brzostek ER, Darby BA, Kramer MA, Phillips RP (2015) Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Glob Chang Biol 21(5):2082–94
Fontaine S, Bardoux G, Abbadie L, Mariotti A (2004) Carbon input to soil may decrease soil carbon content. Ecol Lett 7:314–320
Fontaine S, Henault C, Aamor A, Bdioui N, Bloor JMG, Maire V, Mary B, Revaillot S, Maron PA (2011) Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol Biochem 43:86–96
Freschet GT, Aerts R, Cornelissen JHC (2012) A plant economics spectrum of litter decomposability. Funct Ecol 26:56–65
Freschet GT, Cornwell WK, Wardle DA, Elumeeva TG, Liu W, Jackson BG, Onipchenko VG, Soudzilovskaia NA, Tao J, Cornelissen JHC (2013) Linking litter decomposition of above- and below-ground organs to plant-soil feedbacks worldwide. J Ecol 101:943–952
Gargaglione V, Bahamonde HA, Peri PL (2019) Decomposition and nutrient release of grass and tree fine roots along an environmental gradient in southern patagonia. Austral Ecol 44:276–289
Gill RA, Jackson RB (2000) Global patterns of root turnover for terrestrial ecosystems. New Phytol 147:13–31
Guyonnet JP, Cantarel AAM, Simon L, Haichar FeZ (2018) Root exudation rate as functional trait involved in plant nutrient-use strategy classification. Ecol Evol 8:8573–8581
Han M, Sun L, Gan D, Fu L, Zhu B (2020) Root functional traits are key determinants of the rhizosphere effect on soil organic matter decomposition across 14 temperate hardwood species. Soil Biol Biochem 151:108019
Henneron L, Cros C, Picon-Cochard C, Rahimian V, Fontaine S (2020) Plant economic strategies of grassland species control soil carbon dynamics through rhizodeposition. J Ecol 108:528–545
Henneron L, Kardol P, Wardle DA, Cros C, Fontaine S (2020) Rhizosphere control of soil nitrogen cycling: A key component of plant economic strategies. New Phytol 228:1269–1282
Hinsinger P, Bengough AG, Vetterlein D, Young IM (2009) Rhizosphere: Biophysics, biogeochemistry and ecological relevance. Plant Soil 321:117–152
Hopkins F, Gonzalez-Meler MA, Flower CE, Lynch DJ, Czimczik C, Tang J, Subke J-A (2013) Ecosystem-level controls on root-rhizosphere respiration. New Phytol 199:339–351
Huo CF, Luo YQ, Cheng WX (2017) Rhizosphere priming effect: A meta-analysis. Soil Biol Biochem 111:78–84
Hutsch BW, Augustin J, Merbach W (2002) Plant rhizodeposition - an important source for carbon turnover in soils. J Plant Nutr Soil Sci 165:397–407
Jiang ZH, Liu YZ, Yang JP, Brookes PC, Gunina A (2021) Rhizosphere priming regulates soil organic carbon and nitrogen mineralization: The significance of abiotic mechanisms. Geoderma 385:114877
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: Carbon trading at the soil-root interface. Plant Soil 321:5–33
Kastovska E, Santruckova H (2007) Fate and dynamics of recently fixed c in pasture plant-soil system under field conditions. Plant Soil 300:61–69
Kastovska E, Santruckova H (2011) Comparison of uptake of different n forms by soil microorganisms and two wet-grassland plants: A pot study. Soil Biol Biochem 43:1285–1291
Kastovska E, Edwards K, Picek T, Šantrůčková H (2014) A larger investment into exudation by competitive versus conservative plants is connected to more coupled plant–microbe n cycling. Biogeochemistry 122:47–59
Kastovska E, Edwards K, Santruckova H (2017) Rhizodeposition flux of competitive versus conservative graminoid: Contribution of exudates and root lysates as affected by n loading. Plant Soil 412:331–344
Kirkby CA, Richardson AE, Wade LJ, Passioura JB, Batten GD, Blanchard C, Kirkegaard JA (2014) Nutrient availability limits carbon sequestration in arable soils. Soil Biol Biochem 68:402–409
Knops JMH, Bradley KL, Wedin DA (2002) Mechanisms of plant species impacts on ecosystem nitrogen cycling. Ecol Lett 5:454–466
Kuzyakov Y (2002) Review: Factors affecting rhizosphere priming effects. J Plant Nutr Soil Sci 165:382–396
Kuzyakov Y (2002) Separating microbial respiration of exudates from root respiration in non-sterile soils: A comparison of four methods. Soil Biol Biochem 34:1621–1631
Kuzyakov Y, Cheng W (2001) Photosynthesis controls of rhizosphere respiration and organic matter decomposition. Soil Biol Biochem 33:1915–1925
Kuzyakov Y, Cheng W (2004) Photosynthesis controls of co2 efflux from maize rhizosphere. Plant Soil 263:85–99
Kuzyakov Y, Gavrichkova O (2010) Review: Time lag between photosynthesis and carbon dioxide efflux from soil: A review of mechanisms and controls. Glob Change Biol 16:3386–3406
Kuzyakov Y, Xu XL (2013) Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytol 198:656–669
Legay N, Baxendale C, Grigulis K, Krainer U, Kastl E, Schloter M, Bardgett RD, Arnoldi C, Bahn M, Dumont M, Poly F, Pommier T, Clement JC, Lavorel S (2014) Contribution of above- and below-ground plant traits to the structure and function of grassland soil microbial communities. Ann Bot 114:1011–1021
Liu L, Greaver TL (2010) A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecol Lett 13:819–828
Lloyd DA, Ritz K, Paterson E, Kirk GJD (2016) Effects of soil type and composition of rhizodeposits on rhizosphere priming phenomena. Soil Biol Biochem 103:512–521
Ma XM, Razavi BS, Holz M, Blagodatskaya E, Kuzyakov Y (2017) Warming increases hotspot areas of enzyme activity and shortens the duration of hot moments in the root-detritusphere. Soil Biol Biochem 107:226–233
Marschner P, Marhan S, Kandeler E (2012) Microscale distribution and function of soil microorganisms in the interface between rhizosphere and detritusphere. Soil Biol Biochem 49:174–183
Mason-Jones K, Schmucker N, Kuzyakov Y (2018) Contrasting effects of organic and mineral nitrogen challenge the n-mining hypothesis for soil organic matter priming. Soil Biol Biochem 124:38–46
Mastný J, Kaštovská E, Bárta J, Chroňáková A, Borovec J, Šantrůčková H, Urbanová Z, Edwards RK, Picek T (2018) Quality of doc produced during litter decomposition of peatland plant dominants. Soil Biol Biochem 121:221–230
Mayer J, Buegger F, Jensen ES, Schloter M, Hess J (2003) Estimating n rhizodeposition of grain legumes using a n-15 in situ stem labelling method. Soil Biol Biochem 35:21–28
Mooshammer M, Wanek W, Hammerle I, Fuchslueger L, Hofhansl F, Knoltsch A, Schnecker J, Takriti M, Watzka M, Wild B, Keiblinger KM, Zechmeister-Boltenstern S, Richter A (2014a) Adjustment of microbial nitrogen use efficiency to carbon: Nitrogen imbalances regulates soil nitrogen cycling. Nat Commun 5:3694
Mooshammer M, Wanek W, Zechmeister-Boltenstern S, Richter A (2014b) Stoichiometric imbalances between terrestrial decomposer communities and their resources: Mechanisms and implications of microbial adaptations to their resources. Front Microbiol 5:22
Nguyen C (2003) Rhizodeposition of organic c by plants: Mechanisms and controls. Agronomie 23:375–396
Nottingham AT, Griffiths H, Chamberlain PM, Stott AW, Tanner EVJ (2009) Soil priming by sugar and leaf-litter substrates: A link to microbial groups. Appl Soil Ecol 42:183–190
Pascault N, Ranjard L, Kaisermann A, Bachar D, Christen R, Terrat S, Mathieu O, Leveque J, Mougel C, Henault C, Lemanceau P, Pean M, Boiry S, Fontaine S, Maron PA (2013) Stimulation of different functional groups of bacteria by various plant residues as a driver of soil priming effect. Ecosystems 16:810–822
Pausch J, Kuzyakov Y (2018) Carbon input by roots into the soil: Quantification of rhizodeposition from root to ecosystem scale. Glob Change Biol 24:1–12
Poll C, Marhan S, Ingwersen J, Kandeler E (2008) Dynamics of litter carbon turnover and microbial abundance in a rye detritusphere. Soil Biol Biochem 40:1306–1321
Rasse DP, Rumpel C, Dignac MF (2005) Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 269:341–356
Santruckova H, Bird MI, Lloyd J (2000) Microbial processes and carbon-isotope fractionation in tropical and temperate grassland soils. Funct Ecol 14:108–114
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: A theoretical model. Soil Biol Biochem 35:549–563
Schmidt MWI, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, Koegel-Knabner I, Lehmann J, Manning DAC, Nannipieri P, Rasse DP, Weiner S, Trumbore SE (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56
Shahzad T, Chenu C, Genet P, Barot S, Perveen N, Mougin C, Fontaine S (2015) Contribution of exudates, arbuscular mycorrhizal fungi and litter depositions to the rhizosphere priming effect induced by grassland species. Soil Biol Biochem 80:146–155
Silver WL, Miya RK (2001) Global patterns in root decomposition: Comparisons of climate and litter quality effects. Oecologia 129:407–419
Sinsabaugh RL (2010) Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol Biochem 42:391–404
Sinsabaugh RL, Hill BH, Shah JJF (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462:795–798
Sokol NW, Kuebbing SE, Karlsen-Ayala E, Bradford MA (2019) Evidence for the primacy of living root inputs, not root or shoot litter, in forming soil organic carbon. New Phytol 221:233–246
Solly E, Schöning I, Boch S, Kandeler E, Marhan S, Michalzik B, Müller J, Zscheischler J, Trumbore S, Schrumpf M (2014) Factors controlling decomposition rates of fine root litter in temperate forests and grasslands. Plant Soil 382:203–218
Spohn M, Kuzyakov Y (2014) Spatial and temporal dynamics of hotspots of enzyme activity in soil as affected by living and dead roots-a soil zymography analysis. Plant Soil 379:67–77
Sun K, Luke McCormack M, Li L, Ma Z, Guo D (2016) Fast-cycling unit of root turnover in perennial herbaceous plants in a cold temperate ecosystem. Sci Rep 6:19698
Tjoelker MG, Craine JM, Wedin D, Reich PB, Tilman D (2005) Linking leaf and root trait syndromes among 39 grassland and savannah species. New Phytol 167:493–508
Treseder KK (2008) Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies. Ecol Lett 11:1111–1120
Vale M, Nguyen C, Dambrine E, Dupouey JL (2005) Microbial activity in the rhizosphere soil of six herbaceous species cultivated in a greenhouse is correlated with shoot biomass and root c concentrations. Soil Biol Biochem 37:2329–2333
Zamanian K, Zarebanadkouki M, Kuzyakov Y (2018) Nitrogen fertilization raises co2 efflux from inorganic carbon: A global assessment. Glob Change Biol 24:2810–2817
Zhu B, Gutknecht JLM, Herman DJ, Keck DC, Firestone MK, Cheng WX (2014) Rhizosphere priming effects on soil carbon and nitrogen mineralization. Soil Biol Biochem 76:183–192
Acknowledgements
We gratefully acknowledge funding by the Czech Science Foundation (GACR, project 19-17139S). YK thanks for the support by the Tyumen Oblast Government (No. 89-DON 1), the Program of Competitive Growth of Kazan Federal University and “RUDN University Strategic Academic Leadership program”. We thank our technicians Ondra Žampach, Dan Vaněk, and Lenka Čapková for their help with destructive samplings and laboratory analyses and Láďa Marek for isotopic data measurement. We thank Ryan Scott and Gabriela Scott Zemanová for the proofreading.
Funding
Funding of EK and JC-H by the project of Czech Science Foundation GACR (No. 19-17139S), funding of YK by the project of Tyumen Oblast Government (No. 89-DON 1), the Program of Competitive Growth of Kazan Federal University and “RUDN University Strategic Academic Leadership program”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest/Competing interests
We declare no conflicts of interests.
Additional information
Responsible Editor: Hans Lambers.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 83.5 KB)
Rights and permissions
About this article
Cite this article
Kaštovská, E., Cardenas-Hernandez, J. & Kuzyakov, Y. Priming effects in the rhizosphere and root detritusphere of two wet-grassland graminoids. Plant Soil 472, 105–126 (2022). https://doi.org/10.1007/s11104-021-05191-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-021-05191-6