Abstract
Aims
Changes in plant net primary production due to climate change can influence aboveground and belowground litter inputs to forest soils. We aim to examine the effects of such changes on soil carbon in subtropical forest ecosystems where these effects have not been thoroughly investigated.
Methods
We manipulated aboveground litter inputs and excluded roots in a factorial design, and measured the effects of each treatment and their interactions on soil carbon (C) and soil microbial community structure.
Results
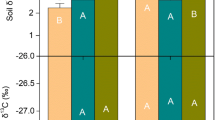
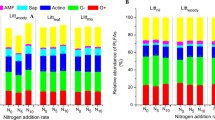
After only 3 years of treatment, aboveground litter addition and root exclusion respectively caused 9% and 21% reductions in soil C concentration in the 0–10 cm soil, likely through different mechanisms. The reduction of soil C with aboveground litter addition was attributed to a priming effect, while reduced root-derived C inputs were likely the cause of the C reduction associated with root exclusion. PLFA analysis showed that both aboveground and belowground litter manipulations reduced Gram-positive bacteria biomass (by 30%–58%) compare to the control, but only root exclusion significantly reduced the actinobacteria biomarkers (by 46%–58%). Fungi, arbuscular mycorrhizal fungi, and ratios of Gram-negative to Gram-positive bacteria and bacteria to fungi did not differ among treatments.
Conclusion
Our results show that root-derived C inputs exert a stronger control on soil C concentrations and microbial community structures than aboveground litter does in subtropical natural forest soils. Our study also highlights that that both increases in aboveground litter and decreases in belowground C input to soil can lead to reduced soil C.


Similar content being viewed by others
References
Bai W, Wan S, Niu S, Liu W, Chen Q, Wang Q, Zhang W, Han X, Li L (2010) Increased temperature and precipitation interact to affect root production, mortality, and turnover in a temperate steppe: implications for ecosystem C cycling. Glob Chang Biol 16:1306–1316
Bowden RD, Deem L, Plante AF, Peltre C, Nadelhoffer K, Lajtha K (2014) Litter input controls on soil carbon in a temperate deciduous Forest. Soil Sci Soc Am J 78:S66–S75
Brant JB, Myrold DD, Sulzman EW (2006a) Root controls on soil microbial community structure in forest soils. Oecologia 148:650–659
Brant JB, Sulzman EW, Myrold DD (2006b) Microbial community utilization of added carbon substrates in response to long-term carbon input manipulation. Soil Biol Biochem 38:2219–2232
Carter MR, Gregorich EG (2006) Soil sampling and methods of analysis. CRC Press, Taylor & Francis Group, Boca Raton
Chen X, Chen HY (2018) Global effects of plant litter alterations on soil CO2 to the atmosphere. Glob Chang Biol 24:3462–3471
Cleveland CC, Townsend AR (2006) Nutrient additions to a tropical rain forest drive substantial soil carbon dioxide losses to the atmosphere. Proc Natl Acad Sci U S A 103:10316–10321
Cleveland CC, Nemergut DR, Schmidt SK, Townsend AR (2007) Increases in soil respiration following labile carbon additions linked to rapid shifts in soil microbial community composition. Biogeochemistry 82:229–240
Cusack DF, Halterman SM, Tanner EVJ, Wright SJ, Hockaday W, Dietterich LH, Turner BL (2018) Decadal-scale litter manipulation alters the biochemical and physical character of tropical forest soil carbon. Soil Biol Biochem 124:199–209
Davidson EA, Savage K, Bolstad P, Clark DA, Curtis PS, Ellsworth DS, Hanson PJ, Law BE, Luo Y, Pregitzer KS (2002) Belowground carbon allocation in forests estimated from litterfall and IRGA-based soil respiration measurements. Agric For Meteorol 113:39–51
Doughty CE, Metcalfe D, Girardin C, Amézquita FF, Cabrera DG, Huasco WH, Silva-Espejo J, Araujo-Murakami A, da Costa M, Rocha W (2015) Drought impact on forest carbon dynamics and fluxes in Amazonia. Nature 519:78–82
Drake JE, Gallet-Budynek A, Hofmockel KS, Bernhardt ES, Billings SA, Jackson RB, Johnsen KS, Lichter J, McCarthy HR, McCormack ML, Moore DJP, Oren R, Palmroth S, Phillips RP, Pippen JS, Pritchard SG, Treseder KK, Schlesinger WH, DeLucia EH, Finzi AC (2011) Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecol Lett 14:349–357
Epron D, Nouvellon Y, Ryan MG (2012) Introduction to the invited issue on carbon allocation of trees and forests. Tree Physiol 32:639–643
Fang HJ, Yu GR, Cheng SL, Mo JM, Yan JH, Li S (2009) 13C abundance, water-soluble and microbial biomass carbon as potential indicators of soil organic carbon dynamics in subtropical forests at different successional stages and subject to different nitrogen loads. Plant Soil 320:243–254
Fekete I, Kotroczó Z, Varga C, Nagy PT, Várbíró G, Bowden RD, Tóth JA, Lajtha K (2014) Alterations in forest detritus inputs influence soil carbon concentration and soil respiration in a central-European deciduous forest. Soil Biol Biochem 74:106–114
Finzi AC, Norby RJ, Calfapietra C, Gallet-Budynek A, Gielen B, Holmes WE, Hoosbeek MR, Iversen CM, Jackson RB, Kubiske ME (2007) Increases in nitrogen uptake rather than nitrogen-use efficiency support higher rates of temperate forest productivity under elevated CO2. Proc Natl Acad Sci U S A 104:14014–14019
Frostegård Å, Tunlid A, Bååth E (2011) Use and misuse of PLFA measurements in soils. Soil Biol Biochem 43:1621–1625
Gatti L, Gloor M, Miller J, Doughty C, Malhi Y, Domingues L, Basso L, Martinewski A, Correia C, Borges V (2014) Drought sensitivity of Amazonian carbon balance revealed by atmospheric measurements. Nature 506:76–80
Gorka S, Dietrich M, Mayerhofer W, Gabriel R, Wiesenbauer J, Martin V, Zheng Q, Imai B, Prommer J, Weidinger M, Schweiger P, Eichorst SA, Wagner M, Richter A, Schintlmeister A, Woebken D, Kaiser C (2019) Rapid transfer of plant photosynthates to soil bacteria via ectomycorrhizal hyphae and its interaction with nitrogen availability. Front Microbiol 10:168. https://doi.org/10.3389/fmicb.2019.00168
Griffiths B, Ritz K, Ebblewhite N, Dobson G (1998) Soil microbial community structure: effects of substrate loading rates. Soil Biol Biochem 31:145–153
Hasibeder R, Fuchslueger L, Richter A, Bahn M (2015) Summer drought alters carbon allocation to roots and root respiration in mountain grassland. New Phytol 205:1117–1127
Hickler T, Smith B, Prentice IC, Mjöfors K, Miller P, Arneth A, Sykes MT (2008) CO2 fertilization in temperate FACE experiments not representative of boreal and tropical forests. Glob Chang Biol 14:1531–1542
Joergensen RG (1996) The fumigation-extraction method to estimate soil microbial biomass: calibration of the k EC value. Soil Biol Biochem 28:25–31
Jones D, Willett V (2006) Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol Biochem 38:991–999
Keenan TF, Gray J, Friedl MA, Toomey M, Bohrer G, Hollinger DY, Munger JW, O’Keefe J, Schmid HP, Wing IS, Yang B, Richardson AD (2014) Net carbon uptake has increased through warming-induced changes in temperate forest phenology. Nat Clim Chang 4:598–604
Khamna S, Yokota A, Lumyong S (2009) Actinomycetes isolated from medicinal plant rhizosphere soils: diversity and screening of antifungal compounds, indole-3-acetic acid and siderophore production. World J Microbiol Biotechnol 25:649
Kuzyakov Y, Friedel J, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498
Lajtha K, Bowden RD, Nadelhoffer K (2014a) Litter and root manipulations provide insights into soil organic matter dynamics and stability. Soil Sci Soc Am J 78:S261
Lajtha K, Townsend KL, Kramer MG, Swanston C, Bowden RD, Nadelhoffer K (2014b) Changes to particulate versus mineral-associated soil carbon after 50 years of litter manipulation in forest and prairie experimental ecosystems. Biogeochemistry 119:341–360
Lajtha K, Bowden RD, Crow S, Fekete I, Kotroczó Z, Plante A, Simpson MJ, Nadelhoffer KJ (2018) The detrital input and removal treatment (DIRT) network: insights into soil carbon stabilization. Sci Total Environ 640:1112–1120
Leff JW, Wieder WR, Taylor PG, Townsend AR, Nemergut DR, Grandy S, Cleveland CC (2012) Experimental litterfall manipulation drives large and rapid changes in soil carbon cycling in a wet tropical forest. Glob Chang Biol 18:2969–2979
Lin KM, Lyu MK, Jiang MH, Chen YM, Li YQ, Chen GS, Xie JS, Yang YY (2017) Improved allometric equations for estimating biomass of the three Castanopsis carlesii H. forest types in subtropical China. New For 48:115–135
Liu C, Westman CJ, Berg B, Kutsch W, Wang GZ, Man R, Ilvesniemi H (2004) Variation in litterfall-climate relationships between coniferous and broadleaf forests in Eurasia. Glob Ecol Biogeogr 13:105–114
Liu XF, Lin TC, Yang ZJ, Vadeboncoeur MA, Lin CF, Xiong DC, Lin WS, Chen GS, Xie JS, Li YQ, Yang YY (2017) Increased litter in subtropical forests boosts soil respiration in natural forests but not plantations of Castanopsis carlesii. Plant Soil 418:141–151
McCarthy AJ, Williams ST (1992) Actinomycetes as agents of biodegradation in the environment-a review. Gene 115:189–192
McKinley V, Peacock A, White D (2005) Microbial community PLFA and PHB responses to ecosystem restoration in tallgrass prairie soils. Soil Biol Biochem 37:1946–1958
Nadelhoffer KJ, Boone RD, Bowden RD, Canary JD, Micks P, Ricca A, Aitkenhead JA, Lajtha K, McDowell WH (2006) The DIRT experiment. Forests in time: the environmental consequences of 1,000 years of change in New England, 300
Nemergut DR, Cleveland CC, Wieder WR, Washenberger CL, Townsend AR (2010) Plot-scale manipulations of organic matter inputs to soils correlate with shifts in microbial community composition in a lowland tropical rain forest. Soil Biol Biochem 42:2153–2160
Nicolardot B, Fauvet G, Cheneby D (1994) Carbon and nitrogen cycling through soil microbial biomass at various temperatures. Soil Biol Biochem 26:253–261
Pan Y, Birdsey RA, Fang J, Houghton R, Kauppi PE, Kurz WA, Phillips OL, Shvidenko A, Lewis SL, Canadell JG (2011) A large and persistent carbon sink in the world’s forests. Science 333:988–993
Paul EA (2014) Soil microbiology and biochemistry, 2nd edn. Academic Press, San Diego
Pisani O, Lin LH, Lun OOY, Lajtha K, Nadelhoffer KJ, Simpson AJ, Simpson MJ (2016) Long-term doubling of litter inputs accelerates soil organic matter degradation and reduces soil carbon stocks. Biogeochemistry 127:1–14
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rasse DP, Rumpel C, Dignac MF (2005) Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 269:341–356
Schaefer DA, Feng W, Zou X (2009) Plant carbon inputs and environmental factors strongly affect soil respiration in a subtropical forest of southwestern China. Soil Biol Biochem 41:1000–1007
Šnajdr J, Valášková V, Merhautová VR, Herinková J, Cajthaml T, Baldrian P (2008) Spatial variability of enzyme activities and microbial biomass in the upper layers of Quercus petraea forest soil. Soil Biol Biochem 40:2068–2075
Soil Survey Staff (2014) Natural Resources Conservation Service 2014 National soil survey handbook. Title 430-VI. U.S. Government Printing Office, Washington D.C. Sec 602
Sokol NW, Bradford MA (2019) Microbial formation of stable soil carbon is more efficient from belowground than aboveground input. Nat Geosci 12:46–53
State Soil Survey Service of China (1998) China soil. China Agricultural Press, Beijing
Subke JA, Hahn V, Battipaglia G, Linder S, Buchmann N, Cotrufo MF (2004) Feedback interactions between needle litter decomposition and rhizosphere activity. Oecologia 139:551–559
Sulzman EW, Brant JB, Bowden RD, Lajtha K (2005) Contribution of aboveground litter, belowground litter, and rhizosphere respiration to total soil CO2 efflux in an old growth coniferous forest. Biogeochemistry 73:231–256
ter Braak CJF, Smilauer P (2012) Canoco reference manual and User's guide: software for ordination (version 5.0). Microcomputer Power, Ithaca
Tveit A, Schwacke R, Svenning MM, Urich T (2013) Organic carbon transformations in high-Arctic peat soils: key functions and microorganisms. ISME J 7:299–311
Vance E, Brookes P, Jenkinson D (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Waldrop MP, Firestone MK (2004) Microbial community utilization of recalcitrant and simple carbon compounds: impact of oak-woodland plant communities. Oecologia 138:275–284
Wan XH, Huang ZQ, He ZM, Yu ZP, Wang MH, Davis MR, Yang YY (2015) Soil C: N ratio is the major determinant of soil microbial community structure in subtropical coniferous and broadleaf forest plantations. Plant Soil 387:103–116
Wang Q, He T, Wang S, Liu L (2013) Carbon input manipulation affects soil respiration and microbial community composition in a subtropical coniferous forest. Agric For Meteorol 178:152–160
Wang Q, Yu Y, He T, Wang Y (2017) Aboveground and belowground litter have equal contributions to soil CO2 emission: an evidence from a 4-year measurement in a subtropical forest. Plant Soil 421:7–17
Whalen JK, Bottomley PJ, Myrold DD (2000) Carbon and nitrogen mineralization from light-and heavy-fraction additions to soil. Soil Biol Biochem 32:1345–1352
Williams ST, Shameemullah M, Watson ET, Mayfield C (1972) Studies on the ecology of actinomycetes in soil-VI. The influence of moisture tension on growth and survival. Soil Biol Biochem 4:215–225
Xu S, Liu L, Sayer E (2013) Variability of above-ground litter inputs alters soil physicochemical and biological processes: a meta-analysis of litterfall manipulation experiments. Biogeosciences 10:7423–7433
Yang Y, Guo J, Chen G, Yin Y, Gao R, Lin C (2009) Effects of forest conversion on soil labile organic carbon fractions and aggregate stability in subtropical China. Plant Soil 323:153–162
Yarwood S, Brewer E, Yarwood R, Lajtha K, Myrold D (2013) Soil microbe active community composition and capability of responding to litter addition after 12 years of no inputs. Appl Environ Microbiol 79:1385–1392
Acknowledgements
This research was jointly supported by Joint Fund for Promotion of Cross-strait Cooperation in Science and Technology (U1505233), National Natural Science Foundation of China (No. 31870601) and National Key Basic Research Program of China (2014CB954003). We are grateful to Cuicui Wei and Xianfeng Li for assistance in soil sampling and laboratory analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Alfonso Escudero.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, X., Lin, TC., Vadeboncoeur, M.A. et al. Root litter inputs exert greater influence over soil C than does aboveground litter in a subtropical natural forest. Plant Soil 444, 489–499 (2019). https://doi.org/10.1007/s11104-019-04294-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04294-5