Abstract
Aims
Understanding the factors that influence the diversity of soybean-nodulating rhizobia is important before doing inoculation. Since studies about this topic in tropical regions are limited, this could lay the groundwork for related research particularly on Bradyrhizobium elkanii.
Methods
To determine the genetic diversity of B. elkanii in different regions, we conducted Polymerase Chain Reaction-Restriction Fragment Length Polymorphism (PCR-RFLP) and sequence analysis of 16S rRNA gene, internal transcribed spacer (ITS) region and rpoB gene. Also, sequence analysis of symbiotic nifD and nodD1 genes was conducted.
Results
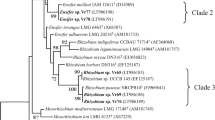
Analysis of the rpoB gene revealed a higher genetic diversity than the ITS region, and possible endemic B. elkanii strains were observed. Meanwhile, no variation was detected among the strains in both nifD and nodD1 phylogenies. Through rpoB gene analysis, variations in the ITS-rpoB type of B. elkanii strains were distinguished and differentiated with that of the closest reference strains. We identified potential soybean inoculants which possess symbiotic efficiency regardless of the Rj genotypes used, suggesting broad host-range of the strains.
Conclusions
We show how the genetic diversity of soybean-nodulating B. elkanii strains in subtropical and tropical regions might be influenced by temperature and soil pH and, provided some insights between the symbiotic genes and Rj genotypes.




Similar content being viewed by others
References
Adhikari D, Kaneto M, Itoh K, Suyama K, Pokharel BB, Gaihre YK (2012) Genetic diversity of soybean-nodulating rhizobia in Nepal in relation to climate and soil properties. Plant Soil 357:131–145
Alam F, Bhuiyan MA, Alam SS, Waghmode TR, Kim PJ, Lee YB (2015) Effect of Rhizobium sp. BARIRGm901 inoculation on nodulation, nitrogen fixation and yield of soybean (Glycine max) genotypes in gray terrace soil. Biosci Biotechnol Biochem 79:1660–1668
Alves BJR, Boddey RM, Urquiaga S (2003) The success of BNF in soybean in Brazil. Plant Soil 252:1–9
Ansari PG, Rao DLN, Pal KK (2013) Diversity and phylogeny of soybean rhizobia in central India. Ann Microbiol 64:1553–1565
Barcellos FG, Menna P, Batista JS, Hungria M (2007) Evidence of horizontal transfer of symbiotic genes from a Bradyrhizobium japonicum inoculant strain to indigenous Diazotrophs Sinorhizobium (Ensifer) fredii and Bradyrhizobium elkanii in a Brazilian savannah soil. Appl Environ Micobiol 73:2635–2643
Bernstein L, MacKenzie AJ, Krantz BA (1955) Salt tolerance of field crops - soybeans. In: United States salinity laboratory report to collaborators. Riverside, CA, pp 35–36
Carrascal OMP, Vanlnsberghe D, Juarez S, Polz MF, Vinuesa P, Gonzalez V (2016) Population genomics of the symbiotic plasmids of sympatric nitrogen-fixing Rhizobium species associated with Phaseolus vulgaris. Environ Microbiol 18:2660–2676
Cole MA, Elkan GH (1973) Transmissible resistance to penicillin G, neomycin, and chloramphenicol in Rhizobium japonicum. Antimicrob Ag Chemother 4:248–253
Degefu T, Wolde-Meskel E, Frostegård Å (2013) Phylogenetic diversity of Rhizobium strains nodulating diverse legume species growing in Ethiopia. Syst Appl Microbiol 36:272–280
Delamuta JRM, Ribeiro RA, Menna P, Bangel EV, Hungria M (2012) Multilocus sequence analysis (MLSA) of Bradyrhizobium strains: revealing high diversity of tropical Diazotrophic symbiotic bacteria. Braz J Microbiol 43:698–710
Delamuta JR, Ribeiro RA, Ormeño-Orrillo E, Melo IS, Martinez-Romero E, Hungria M (2013) Polyphasic evidence supporting the reclassification of Bradyrhizobium japonicum group Ia strains as Bradyrhizobium diazoefficiens sp. nov. Int J Syst Evol Microbiol 63:3342–3351
Devine TE, Kuykendall LD (1996) Host genetic control of symbiosis in soybean (Glycine max L.) Plant Soil 186:173–187
Didelot X, Maiden MCJ (2010) Impact of recombination on bacterial evolution. Trends Microbiol 18:315–322
Germano MG, Menna P, Mostasso FL, Hungria M (2006) RFLP analysis of the rRNA operon of a Brazilian collection of bradyrhizobial strains from 33 legume species. Int J Syst Evol Microbiol 56:217–229
Gordon BR, Klinger CR, Weese DJ, Lau JA, Burke PV, Dentinger BTM, Heath KD (2016) Decoupled genomic elements and the evolution of partner quality in nitrogen-fixing rhizobia. Ecol Evol 6:1317–1327
Guimarães AA, Florentino LA, Almeida KA, Lebbe L, Silva KB, Willems A, Moreira FM (2015) High diversity of Bradyrhizobium strains isolated from several legume species and land uses in Brazilian tropical ecosystems. Syst Appl Microbiol 38:433–441
Gyaneshwar P, Hirsch AM, Moulin L, Chen W, Elliott GN, Bontemps C, de los Santos PE, Gross E, dos Resi FB Jr, Sprent JI, Young JPW, James EK (2011) Legume-nodulating Betaproteobacteria: diversity, host range, and future prospects. MPMI 24:1276–1288
Hiraishi A, Kamagata Y, Nakamura K (1995) Polymerase chain reaction amplification and restriction fragment length polymorphism analysis of 16S rRNA genes from methanogens. J Ferment Bioeng 79:523–529
Htwe AZ, Yamakawa T, Sarr PS, Sakata T (2015) Diversity and distribution of soybean-nodulating bradyrhizobia isolated from major soybean-growing regions in Myanmar. Afr J Microbiol Res 9:2183–2196
Ikeda S, Rallos LEE, Okubo T, Eda S, Inaba S, Mitsui H, Minamisawa K (2008) Microbial community analysis of field-grown soybeans with different nodulation phenotypes. Appl Environ Microbiol 74:5704–5709
Ikeda S, Okubo T, Kaneko T, Inaba S, Maekawa T, Eda S et al (2010) Community shifts of soybean stem-associated bacteria responding to different nodulation phenotypes and N levels. ISME J 4:315–326
Jordan DC (1982) Transfer of Rhizobium japonicum Buchanan 1980 to Bradyrhizobium gen. Nov., a genus of slow-growing, root nodule bacteria from leguminous plants. Int J Syst Bacteriol 32:136–139
Keyser HH, Bohlool BB, Hu TS, Weber DF (1982) Fast-growing rhizobia isolated from root nodules of soybean. Science 215:1631–1632
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Kuykendall LD, Saxena B, Devine TE, Udell SE (1992) Genetic diversity in Bradyrhizobium Jordan 1982 and a proposal for Bradyrhizobium elkanii sp. nov. Can J Microbiol 38:501–505
Laguerre G, Nour SM, Macheret V, Sanjuan J, Drouin P, Amarger N (2001) Classification of rhizobia based on nodC and nifH gene analysis reveals a close phylogenetic relationship among Phaseolus vulgaris symbionts. Microbiol 147:981–993
Ling J, Wang H, Wu P, Li T, Tang Y, Naseer N, Zheng H, Masson-Boivin C, Zong Z, Zhu J (2016) Plant nodulation inducers enhance horizontal gene transfer of Azorhizobium caulinodans symbiosis island. Proc Natl Acad Sci U S A 113:13875–13880
Loureiro M, Kaschuk G, Alberton O, Hungria M (2007) Soybean [Glycine max (L.) Merrill] rhizobial diversity in Brazilian oxisols under various soil, cropping, and inoculation managements. Biol Fertil Soils 43:665–674
Maj D, Wielbo J, Marek-Kozaczuk M, Skorupska A (2010) Response to flavonoids as a factor influencing competitiveness and symbiotic activity of Rhizobium leguminosarum. Microbiol Res 165:50–60
Man CX, Wang H, Chen WF, Sui XH, Wang ET, Chen WX (2008) Diverse rhizobia associated with soybean grown in the subtropical and tropical regions of China. Plant Soil 310:77–87
Manuel PC, Huelgas R, Espanto LH (1986) Adoption of soybean in Lupao, Nueva Ecija, Philippines. UN/ESCAP CGPRT Centre, regional coordination Centre for Research and Development of coarse grains, roots and tuber crops in the humid tropics of Asia and the Pacific. CGPRT no. 7: 62 pp
Martens M, Dawyndt P, Coopman R, Gillis M, De Vos P, Willems A (2008) Advantages of multilocus sequence analysis for taxonomic studies: a case study using 10 housekeeping genes in the genus Ensifer (including former Sinorhizobium). Int J Syst Evol Microbiol 58:200–214
Martinez-Romero E, Caballero-Mellado J (1996) Rhizobium phylogenies and bacterial genetic diversity. Crit Rev Plant Sci 15:113–140
Minami M, Yamakawa T, Yamamoto A, Akao S, Saeki Y (2009) Estimation of nodulation tendency among Rj-genotype soybeans using the bradyrhizobial community isolated from an andosol. Soil Sci Plant Nutr 55:65–72
Minamisawa K, Itakura M, Suzuki M, Ichige K, Isawa T, Yuhashi K, Mitsui H (2002) Horizontal transfer of nodulation genes in soils and microcosms form Bradyrhizobium japonicum to B. elkanii. Microbes Environ 2:82–90
Njira KOW, Nalivata PC, Kanyama-Phiri GY, Lowole MW (2013) An assessment for the need of soybean inoculation with Bradyrhizobium japonicum in some sites of Kasungu district, Central Malawi. Int J Curr Microbiol App Sci 2:60–72
Nguyen MT, Akiyoshi K, Nakatsukasa M, Saeki Y, Yokoyama K (2010) Multiple occupancy of nodules by nodulating rhizobia on field-grown soybeans with attendance of Sinorhizobium spp. Soil Sci Plant Nutr 56:382–389
Peix A, Ramirez-Bahena MH, Velazquez E, Bedmar EJ (2015) Bacterial associations with legumes. Crit Rev Plant Sci 34:17–42
Ramirez-Bahena MH, Peix A, Rivas R, Camacho M, Rodriguez-Navarro DN, Mateos PF, Martinez-Molina E, Willems A, Velazquez E (2009) Bradyrhizobium pachyrhizi sp. nov. and Bradyrhizobium jicamae sp. nov., isolated from effective nodules of Pachyrhizus erosus. Int J Syst Evol Microbiol 59:1929–1934
Redmond M, Batley M, Djordjevic MA, Innes RW, Kuempel PL, Rolfe BG (1986) Flavones induce expression of nodulation genes in Rhizobium. Nature 323:632–634
Risal CP, Yokoyama T, Ohkama-Ohtsu N, Djedidi S, Sekimoto H (2010) Genetic diversity of native soybean bradyrhizobia from different topographical regions along the southern slopes of the Himalayan Mountains in Nepal. Syst Appl Microbiol 33:416–425
Rivas R, Martens M, de Lajudie P, Willems A (2009) Multilocus sequence analysis of the genus Bradyrhizobium. Syst Appl Microbiol 32:101–110
Sameshima R, Isawa T, Sadowsky MJ, Hamada T, Kasai H, Shutsrirung A et al (2003) Phylogeny and distribution of extra-slow-growing Bradyrhizobium japonicum harboring high copy numbers of RSα, RSβ and IS1631. FEMS Microbiol Ecol 44:191–202
Sanz-Sáez A, Heath K, Burke P, Ainsworth E (2015) Inoculation with an enhanced N2O-fixing Bradyrhizobium japonicum strain (USDA110) does not alter soybean (Glycine max Merr.) response to elevated [CO2]. Plant Cell Environ 38:2589–2602
Saeki Y, Akagi I, Takaki H, Nagatomo Y (2000) Diversity of indigenous Bradyrhizobium strains isolated from three different Rj-soybean cultivars in terms of randomly amplified polymorphic DNA and intrinsic antibiotic resistance. Soil Sci Plant Nutr 46:917–926
Saeki Y, Aimi N, Hashimoto M, Tsukamoto S, Kaneko A, Yoshida N et al (2004) Grouping of Bradyrhizobium USDA strains by sequence analysis of 16S rDNA and 16S-23S rDNA internal transcribed spacer region. Soil Sci Plant Nutr 50:517–525
Saeki Y, Aimi N, Tsukamoto S, Yamakawa T, Nagatomo Y, Akao S (2006) Diversity and geographical distribution of indigenous soybean-nodulating bradyrhizobia in Japan. Soil Sci Plant Nutr 52:418–426
Saeki Y, Minami M, Yamamoto A, Akao S (2008) Estimation of the bacterial community diversity of soybean-nodulating bradyrhizobia isolated from Rj-genotype soybeans. Soil Sci Plant Nutr 54:718–724
Saeki Y, Ozumi S, Yamamoto A, Umehara Y, Hayashi M, Sigua GC (2010) Changes in population occupancy of bradyrhizobia under different temperature regimes. Microbes Environ 25:309–312
Saeki Y, Shiro S, Tajima T, Yamamoto A, Sameshima-Saito R, Sato T, Yamakawa T (2013) Mathematical ecology analysis of geographical distribution of soybean-Nodulating Bradyrhizobia in Japan. Microbes Environ 28:470–478
Saeki Y, Shiro S (2014) Comparison of soybean-Nodulating Bradyrhizobia community structures along north latitude between Japan and USA, advances in biology and ecology of nitrogen fixation, prof. Takuji Ohyama (Ed.) InTech, pp. 195–224
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Scholla MH, Elkan HG (1984) Rhizobium fredii sp. nov. a fast-growing species that effectively nodulates soybeans. Int J Syst Bacteriol 34:484–486
Sharma M, Srivastava K, Sharma S (2010) Biochemical characterization and metabolic diversity of soybean rhizobia isolated from Malwa region of Central India. Plant Soil Environ 56:375–383
Shiro S, Yamamoto A, Umehara Y, Hayashi M, Yoshida N, Nishiwaki A et al (2012) Effect of Rj genotype and cultivation temperature on the community structure of soybean-nodulating bradyrhizobia. Appl Environ Microbiol 78:1243–1250
Shiro S, Matsuura S, Saiki R, Sigua GC, Yamamoto A, Umehara Y et al (2013) Genetic diversity and geographical distribution of indigenous soybean-nodulating bradyrhizobia in the United States. Appl Environ Microbiol 79:3610–3618
Suzuki K, Oguro H, Yamakawa T, Yamamoto A, Akao S, Saeki Y (2008) Diversity and distribution of indigenous soybean - nodulating rhizobia in the Okinawa islands, Japan. Soil Sci Plant Nutr 54:237–246
Tian CF, Young JPW, Wang ET, Tamimi SM, Chen WX (2010) Population mixing of Rhizobium leguminosarum bv. viciae nodulating Vicia faba: the role of recombination and lateral gene transfer. FEMS Microbiol Ecol 73:563–576
Tsurumaru H, Yamakawa T, Tanaka M, Sakai M (2008) Tn5 mutants of Bradyrhizobium japonicum is-1 with altered compatibility with Rj2-soybean cultivars. Soil Sci. Plant Nutr 54:197–203
van Berkum P, Fuhrmann JJ (2000) Evolutionary relationships among the soybean bradyrhizobia reconstructed from 16S rRNA gene and internally transcribed spacer region sequence divergence. Int J Syst Evol Microbiol 50:2165–2172
Vincent JM (1970) A manual for the practical study of the root-nodule bacteria. Blackwell Scientific, Oxford
Vinuesa P, Rojas-Jiménez K, Contreras-Moreira B, Mahna SK, Prasad BN, Moe H et al (2008) Multilocus sequence analysis for assessment of the biogeography and evolutionary genetics of four Bradyrhizobium species that Nodulate soybeans on the Asiatic continent. Appl Environ Microbiol 74:6987–6996
Vos M, Quince C, Pijl AS, de Hollander M, Kowalchuk GA (2012) A comparison of rpoB and 16S rRNA as markers in pyrosequencing studies of bacterial diversity. PLoS One 7:1–8
Wang J (2016) Analysis of the factors influencing Japan’s soybean import trade: based on gravity model. Am J Ind Bus Manag 6:109–116
Yamakawa T, Hussain AKMA, Ishizuka J (2003) Soybean preference for Bradyrhizobium japonicum for nodulation. Soil Sci Plant Nutr 49:835–841
Yan J, Han XZ, Ji ZJ, Li Y, Wang ET, Xie ZH, Chen WF (2014) Abundance and diversity of soybean-Nodulating rhizobia in black soil are impacted by land use and crop management. Appl Environ Microbiol 80:5394–5402
Yasuda M, Miwa H, Masuda S, Takebayashi Y, Sakakibara H, Okazaki S (2016) Effecter-triggered immunity determines host genotype-specific incompatibility in legume-rhizobium symbiosis. Plant Cell Physiol 57:1791–1800
Young JM (2003) The genus name Ensifer Casida 1982 takes priority over Sinorhizobium Chen et al. 1988, and Sinorhizobium morelense Wang et al. 2002 is a later synonym of Ensifer adhaerens Casida 1982. Is the combination ‘Sinorhizobium adhaerens’ (Casida 1982) Willems et al. 2003 legitimate? Request for an opinion. Int J Syst Evol Microbiol 53:2107–2110
Zaat SA, Schripsema J, Wijffelman CA, van Brussel AAN, Lugtenberg BJJ (1989) Analysis of the major inducers of the Rhizobium nodA promoter from Vicia sativa root exudate and their activity with different nodD genes. Plant Mol Biol 13:175–188
Zhang YM, Li Y Jr, Chen WF, Wang ET, Tian CF, Li QQ, Zhang YZ, Sui XH, Chen WX (2011) Biodiversity and biogeography of rhizobia associated with soybean plants grown in the North China plain. Appl Environ Microbiol 77:6331–6342
Zhao L, Fan M, Zhang D, Yang R, Zhang F, Xu L, Wei X, Shen Y, Wei G (2014) Distribution and diversity of rhizobia associated with wild soybean (Glycine soja Sieb. & Zucc.) in Northwest China. Syst Appl Microbiol 37:449–456
Acknowledgements
This study was supported by JSPS KAKENHI (Grant-in-Aid for Scientific Research (B) no. 26310313) and the Japanese Government (MEXT) Scholarship program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Katharina Pawlowski.
Electronic supplementary material
Table S1
List of primers used in this study for the PCR amplification and sequence analysis of 16S rRNA, ITS, rpoB, nifD and nodD1. (PDF 73 kb)
Table S2
Shoot and nodule parameters of 19 representative B. elkanii isolates employing three Rj genotypes. Mean comparison was conducted in triplicates only between isolates within the same Rj genotype. (PDF 89 kb)
Table S3
List of accession numbers for selected Bradyrhizobium USDA reference strains and isolates from the sequence analysis of 16S rRNA gene, 16S–23S rRNA gene ITS region, rpoB housekeeping gene and symbiotic genes nifD and nodD1. (PDF 79 kb)
Figure S1
Indication of the usefulness of rpoB-RFLP analysis as shown by the similarity in the phylogeny and RFLP band patterns. (PDF 58 kb)
Figure S2
Phylogenetic tree based on sequence analysis of 16S rRNA gene. The tree was constructed using the Neighbor- Joining method with the Kimura 2-parameter (K2P) distance correlation model and 1000 bootstrap replications in MEGA v.7 software. (PDF 138 kb)
Figure S3
Phylogenetic tree based on sequence analysis of (A) nifD gene and (B) nodD1 gene. The tree was constructed using the Neighbor-Joining method with the Kimura 2-parameter (K2P) distance correlation model and 1000 bootstrap replications in MEGA v.7 software. The first letter of isolates’ name indicates the location as follows: H - Kumamoto; O - Okinawa; P - C. Luzon, Philippines. (PDF 235 kb)
Rights and permissions
About this article
Cite this article
Mason, M.L.T., Matsuura, S., Domingo, A.L. et al. Genetic diversity of indigenous soybean-nodulating Bradyrhizobium elkanii from southern Japan and Nueva Ecija, Philippines. Plant Soil 417, 349–362 (2017). https://doi.org/10.1007/s11104-017-3263-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3263-4