Abstract
Background and aims
The majority of terrestrial plant species associate with arbuscular mycorrhizal (AM) fungi, to exchange carbon compounds with nutrients. However, the factors that determine the composition of AM fungal communities in individual plant roots remain poorly understood. We hypothesized that AM fungal communities are simultaneously influenced by environmental conditions, such as light availability, and the photosynthetic capacity of host plant species.
Methods
We sampled individuals of shade-tolerant and shade-avoidant plant species, growing in the presence and absence of forest canopy, representing conditions of low and high light availability. We recorded photosynthetic parameters, shoot biomass and root AM fungal colonisation of these plant individuals and used 454-sequencing to characterise AM fungal communities in the roots of these plants.
Results
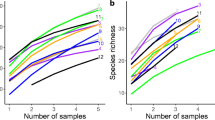
Shade-avoidant plant species increased their photosynthetic capacity more than shade-tolerant plant species as a response to increased light availability due to canopy removal. Root AM fungal colonisation of all plants was higher when the forest canopy was absent, but canopy status had little influence on AM fungal richness in plant roots. The composition of AM fungal communities associating with shade-tolerant plants was significantly influenced by canopy status, while a less pronounced difference was observed among shade-avoidant plants.
Conclusions
We suggest that both environmental conditions and the ability of plant species to exploit available resources determine the dynamics of mutualistic associations between host plant species and AM fungal taxa.


Similar content being viewed by others
References
Aavik T, Püssa K, Roosaluste E, Moora M (2009) Vegetation change in boreonemoral forest during succession ― trends in species composition, richness and differentiation diversity. Ann Bot Fennici 46:326–335. doi:10.5735/085.046.0408
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46. doi:10.1111/j.1442-9993.2001.01070.pp.x
Anderson MJ, Ellingsen KE, McArdle BH (2006) Multivariate dispersion as a measure of beta diversity. Ecol Lett 9:683–693. doi:10.1111/j.1461-0248.2006.00926.x
Avio L, Pellegrino E, Bonari E, Giovannetti M (2006) Functional diversity of arbuscular mycorrhizal fungal isolates in relation to extraradical mycelial networks. New Phytol 172:347–357. doi:10.1111/j.1469-8137.2006.01839.x
Chagnon P-L, Bradley RL, Maherali H, Klironomos JN (2013) A trait-based framework to understand life history of mycorrhizal fungi. Trends Plant Sci 18:484–491. doi:10.1016/j.tplants.2013.05.001
Curtis WF (1984) Photosynthetic potential of sun and shade Viola species. Can J Bot 62:1273–1278. doi:10.1139/b84-171
Davies GM, Gray A (2015) Don't let spurious accusations of pseudoreplication limit our ability to learn from natural experiments (and other messy kinds of ecological monitoring. Ecol Evol 5:5295–5304. doi:10.1002/ece3.1782
Davison J, Öpik M, Daniell TJ, Moora M, Zobel M (2011) Arbuscular mycorrhizal fungal communities in plant roots are not random assemblages. FEMS Microbiol Ecol 78:103–115. doi:10.1111/j.1574-6941.2011.01103.x
Davison J, Öpik M, Zobel M, Vasar M, Metsis M, Moora M (2012) Communities of arbuscular mycorrhizal fungi detected in forest soil are spatially heterogeneous but do not vary throughout the growing season. PLoS One 7. doi:10.1371/journal.pone.0041938
Davison J, Moora M, Öpik M, Adholeya A, Ainsaar L, Bâ A, Burla S, Diedhiou A, Hiiesalu I, Jairus T, Johnson NC, Kane A, Koorem K, Kochar M, Ndiaye C, Pärtel M, Reier Ü, Saks Ü, Singh R, Vasar M, Zobel M (2015) Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism. Science 349:970–973. doi:10.1126/science.aab1161
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecological Monogr 67:345–366. doi:10.1890/0012–9615(1997)067[0345:SAAIST]2.0.CO;2
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200. doi:10.1093/bioinformatics/btr381
Ellenberg H, Weber, HE, Düll, R, Wirth, V, Werner, W, Paulißen, D (1991) Zeigerwerte von pflanzen in Mitteleuropa
Engelmoer DJ, Kiers ET (2014) Host diversity affects the abundance of the extraradicalarbuscular mycorrhizal network. New Phytol 205:1485–1491. doi:10.1111/nph.13086
Engelmoer DJ, Behm JE, Kiers ET (2014) Intense competition between arbuscular mycorrhizal mutualists in an in vitro root microbiome negatively affects total fungal abundance. Mol Ecol 23:1584–1593. doi:10.1111/mec.12451
Estonian Weather Service (2016) Climate normals. http://www.ilmateenistus.ee/kliima/kliimanormid/ohutemperatuur/?lang=en
Fellbaum CR, Mensah JA, Cloos AJ, Strahan GE, Pfeffer PE, Kiers ET, Bücking H (2014) Fungal nutrient allocation in common mycorrhizal networks is regulated by the carbon source strength of individual host plants. New Phytol 203:646–656. doi:10.1111/nph.12827
Füzy A, Bothe H, Molnar E, Biro B (2014) Mycorrhizal symbiosis effects on growth of chalk false-brome (Brachypodium Pinnatum) are dependent on the environmental light regime. J Plant Physiol 171:1–6. doi:10.1016/j.jplph.2013.11.002
Gehring CA (2003) Growth responses to arbuscular mycorrhizae by rain forest seedlings vary with light intensity and tree species. Plant Ecol 167:127–139. doi:10.1023/a:1023989610773
Gommers CM, Visser EJ, St Onge KR, Voesenek LA, Pierik R (2013) Shade tolerance: when growing tall is not an option. Trends Plant Sci 18:65–71. doi:10.1016/j.tplants.2012.09.008
Grilli G, Urcelay C, Galetto L, Davison J, Vasar M, Saks Ü, Jairus T, Öpik M (2015) The composition of arbuscular mycorrhizal fungal communities in the roots of a ruderal forb is not related to the forest fragmentation process. Environ Microbiol 17:2709–2720. doi:10.1111/1462–2920.12623
Hart MM, Reader RJ (2002) Host plant benefit from association with arbuscular mycorrhizal fungi: variation due to differences in size of mycelium. Biol Fert. Soils 36:357–366. doi:10.1007/s00374-002-0539-4
Heinemeyer A, Ridgway KP, Edwards EJ, Benham DG, Young JPW, Fitter AH (2004) Impact of soil warming and shading on colonization and community structure of arbuscular mycorrhizal fungi in roots of a native grassland community. Glob Change Biol 10:52–64. doi:10.1111/j.1365-2486.2003.00713.x
Johnson NC (2010) Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol 185:631–647. doi:10.1111/j.1469-8137.2009.03110.x
Johnson N, Graham JH, Smith F (1997) Functioning of mycorrhizal associations along the mutualism–parasitism continuum*. New Phytol 135: 575–585. doi: 10.1046/j.1469-8137.1997.00729.x
Johnson NC, Wilson GW, Wilson JA, Miller RM, Bowker MA (2015) Mycorrhizal phenotypes and the law of the minimum. New Phytol 205:1473–1484. doi:10.1111/nph.13172
Kaschuk G, Kuyper TW, Leffelaar PA, Hungria M, Giller KE (2009) Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biol Biochem 41:1233–1244. doi:10.1016/j.soilbio.2009.03.005
Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO (2010) Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26:1463–1464. doi:10.1093/bioinformatics/btq166
Kiers ET, Duhamel M, Beesetty Y, Mensah JA, Franken O, Verbruggen E, Fellbaum CR, Kowalchuk GA, Hart MM, Bago A (2011) Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 333:880–882. doi:10.1126/science.1208473
Klotz S, Kühn I, Durka W, Briemle G (2002) BIOLFLOR: Eine Datenbank mit biologisch-ökologischen Merkmalen zur Flora von Deutschland. Bundesamt für Naturschutz Bonn
Knegt B, Jansa J, Franken O, Engelmoer DJP, Werner GDA, Bücking H, Kiers ET (2014) Host plant quality mediates competition between arbuscular mycorrhizal fungi. Fungal Ecol. 20:233–240. doi:10.1016/j.funeco.2014.09.011
Koorem K, Gazol A, Öpik M, Moora M, Saks Ü, Uibopuu A, Sõber V, Zobel M (2014) Soil nutrient content influences the abundance of soil microbes but not plant biomass at the small-scale. PLoS One 9:e91998. doi:10.1371/journal.pone.0091998
Koske R, Gemma J (1989) A modified procedure for staining roots to detect VA mycorrhizas. Mycol Res 92:486–488. doi:10.1016/S0953-7562(89)80195-9
Kull O (2002) Acclimation of photosynthesis in canopies: models and limitations. Oecologia 133:267–279. doi:10.1007/s00442-002-1042-1
Lee J, Lee S, Young JPW (2008) Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbiol Ecol 65:339–349. doi:10.1111/j.1574-6941.2008.00531.x
Lekberg Y, Koide RT (2013) Integrating physiological, community, and evolutionary perspectives on the arbuscular mycorrhizal symbiosis 1. Botany 92:241–251. doi:10.1139/cjb-2013-0182
Lendenmann M, Thonar C, Barnard RL, Salmon Y, Werner RA, Frossard E, Jansa J (2011) Symbiont identity matters: carbon and phosphorus fluxes between Medicago Truncatula and different arbuscular mycorrhizal fungi. Mycorrhiza 21:689–702. doi:10.1007/s00572-011-0371-5
Liu Y, Mao L, Li J, Shi G, Jiang S, Ma X, An L, Du G, Feng H (2014) Resource availability differentially drives community assemblages of plants and their root-associated arbuscular mycorrhizal fungi. Plant Soil 386:341–355. doi:10.1007/s11104–014-2261-z
Mangan SA, Herre EA, Bever JD (2010) Specificity between Neotropical tree seedlings and their fungal mutualists leads to plant–soil feedback. Ecology 91: 2594–2603. doi: 10.1890/09-0396.1.
McGonigle T, Miller M, Evans D, Fairchild G, Swan J (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501. doi:10.1111/j.1469-8137.1990.tb00476.x
Moora M, Daniell T, Kalle H, Liira J, Püssa K, Roosaluste E, Öpik M, Wheatley R, Zobel M (2007) Spatial pattern and species richness of boreonemoral forest understorey and its determinants - a comparison of diffirently managed forests. For Ecol Manag 250:64–70. doi:10.1016/j.foreco.2007.03.010
Moora M, Davison J, Öpik M, Metsis M, Saks Ü, Jairus T, Vasar M, Zobel M (2014) Anthropogenic land use shapes the composition and phylogenetic structure of soil arbuscular mycorrhizal fungal communities. FEMS Microbiol Ecol 90:609–621. doi:10.1111/1574-6941.12420
Ohsowski BM, Zaitsoff PD, Öpik M, Hart MM (2014) Where the wild things are: looking for uncultured Glomeromycota. New Phytol 204:171–179. doi:10.1111/nph.12894
Öpik M, Moora M, Zobel M, Saks Ü, Wheatley R, Wright F, Daniell T (2008) High diversity of arbuscular mycorrhizal fungi in a boreal herb-rich coniferous forest. New Phytol 179:867–876. doi:10.1111/j.1469-8137.2008.02515.x
Öpik M, Metsis M, Daniell TJ, Zobel M, Moora M (2009) Large-scale parallel 454 sequencing reveals host ecological group specificity of arbuscular mycorrhizal fungi in a boreonemoral forest. New Phytol 184:424–437. doi:10.1111/j.1469-8137.2009.02920.x
Öpik M, Vanatoa A, Vanatoa E, Moora M, Davison J, Kalwij JM, Reier Ü, Zobel M (2010) The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota. New Phytol 188:223–241. doi:10.1111/j.1469-8137.2010.03334.x
Öpik M, Zobel M, Cantero JJ, Davison J, Facelli JM, Hiiesalu I, Jairus T, Kalwij JM, Koorem K, Leal ME, Liira J, Metsis M, Neshataeva V, Paal J, Phosri C, Põlme S, Reier Ü, Saks Ü, Schimann H, Thiery O, Vasar M, Moora M (2013) Global sampling of plant roots expands the described molecular diversity of arbuscular mycorrhizal fungi. Mycorrhiza 23:411–430. doi:10.1007/s00572-013-0482-2
Öpik M, Davison J, Moora M, Zobel M (2014) DNA-based detection and identification of Glomeromycota: the virtual taxonomy of environmental sequences. Botany 92:135–147. doi:10.1139/cjb-2013-0110
Pinheiro, JC, Bates, DM (2000) Mixed-effects models in S and S-PLUS. Springer
Powell JR, Parrent JL, Hart MM, Klironomos JN, Rillig MC, Maherali H (2009) Phylogenetic trait conservatism and the evolution of functional trade-offs in arbuscular mycorrhizal fungi. Proc Roy Soc B-Biol Sci 276:4237–4245. doi:10.1098/rspb.2009.1015
Shi G, Liu Y, Johnson NC, Olsson PA, Mao L, Cheng G, Jiang S, An L, Du G, Feng H (2014) Interactive influence of light intensity and soil fertility on root-associated arbuscular mycorrhizal fungi. Plant Soil 378:173–188. doi:10.1007/s11104–014-2022-z
Simon L, Lalonde M, Bruns T (1992) Specific amplification of 18S fungal ribosomal genes from vesicular-arbuscular endomycorrhizal fungi colonizing roots. Appl Environ Microbiol 58: 291–295
Smith, SE, Read, DJ (2010) Mycorrhizal symbiosis. Academic press
Tester M, Smith FA, Smith SE (1985) Phosphate inflow into Trifolium Subterraneum L.: effects of photon irradiance and mycorrhizal infection. Soil Biol Biochem 17:807–810. doi:10.1016/0038-0717(85)90137-3
van der Heijden MGA, Scheublin TR (2007) Functional traits in mycorrhizal ecology: their use for predicting the impact of arbuscular mycorrhizal fungal communities on plant growth and ecosystem functioning. New Phytol 174:244–250. doi:10.1111/j.1469-8137.2007.02041.x
Wall A (2008) Effect of removal of logging residue on nutrient leaching and nutrient pools in the soil after clearcutting in a Norway spruce stand. For Ecol Manag 256:1372–1383. doi:10.1016/j.foreco.2008.06.044
Werner GD, Kiers ET (2015) Order of arrival structures arbuscular mycorrhizal colonization of plants. New Phytol 205:1515–1524. doi:10.1111/nph.13092
Zobel M, Kalamees R, Püssa K, Roosaluste E, Moora M (2007) Soil seed bank and vegetation in mixed coniferous forest stands with different disturbance regimes. For Ecol Manag 250:71–76. doi:10.1016/j.foreco.2007.03.011
Acknowledgments
We thank Annika Uibopuu for measuring AM fungal colonisation in plant roots and Marina Semchenko for comments on an earlier version of the manuscript. This work was supported by Estonian Research Council (grants IUT20-28, PUTJD78), the European Regional Development Fund (Centre of Excellence EcolChange) and European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement no GA-2010-267243 – PLANT FELLOWS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jeff R. Powell .
Electronic supplementary material
ESM 1
(DOCX 130 kb)
Rights and permissions
About this article
Cite this article
Koorem, K., Tulva, I., Davison, J. et al. Arbuscular mycorrhizal fungal communities in forest plant roots are simultaneously shaped by host characteristics and canopy-mediated light availability. Plant Soil 410, 259–271 (2017). https://doi.org/10.1007/s11104-016-3004-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-3004-0