Abstract
Aims
Arbuscular mycorrhizal fungi (AMF) play a key role in the functioning of agricultural ecosystems. Therefore, understanding how the application of fertilizers, a common management practice, affects AMF communities is of major importance. Here we aimed to: (i) experimentally test whether different amounts and forms of phosphorus (P) fertilizer affect AMF diversity and community composition associated with the roots of apple trees (Malus domestica); (ii) identify differences in tolerance to P fertilization between AMF taxa.
Methods
We used 454-pyrosequencing of the small subunit rRNA gene amplicons to quantify AMF diversity and community composition in root samples obtained from a three year field experiment, with two inorganic, three slow-release P fertilization and one control treatment.
Results
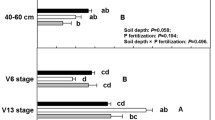
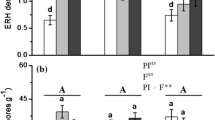
The slow-release fertilizer treatments showed significantly higher AMF richness and differed in community composition compared to the inorganic fertilizer treatments. The distribution of AMF OTUs showed a significantly nested pattern. Additionally, AMF communities in the inorganic fertilizer treatments were a subset of the communities in the slow-release fertilizer treatments.
Conclusions
We demonstrate that application of slow-release fertilizers promoted AMF diversity in the roots of cultivated apple trees in comparison to the other treatments. The application of inorganic fertilizers elevated levels of plant-available P in the soil and selected only a small subset of abundant AMF, resulting in a lower AMF diversity. This may result in AMF communities dominated by inferior AMF mutualists.


Similar content being viewed by others
References
Alguacil MM, Torrecillas E (2011) Evidence of differences between the communities of arbuscular mycorrhizal fungi colonizing galls and roots of Prunus persica infected by the root-knot nematode Meloidogyne incognita. Appl Environ Microbiol 77:8656–8661
Alguacil MM, Lozano Z, Campoy MJ, Roldán A (2010) Phosphorus fertilisation management modifies the biodiversity of AM fungi in a tropical savanna forage system. Soil Biol Biochem 42:1114–1122
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Balestrini R, Magurno F, Walker C, Lumini E, Bianciotto V (2010) Cohorts of arbuscular mycorrhizal fungi (AMF) in Vitis vinifera, a typical Mediterranean fruit crop. Environ Microbiol Rep 2:594–604
Beauregard MS, Gauthier MP, Hamel C, Zhang T, Welacky T, Tan CS, St-Arnaud M (2013) Various forms of organic and inorganic P fertilizers did not negatively affect soil-and root-inhabiting AM fungi in a maize–soybean rotation system. Mycorrhiza 23:143–154
Cavallazzi JRP, Filho OK, Stürmer SL, Rygiewicz P, Mendonça MM (2007) Screening and selecting arbuscular mycorrhizal fungi for inoculating micropropagated apple rootstocks in acid soils. Plant Cell Tissue Organ Cult 90:117–129
Chen YL, Zhang X, Ye JS, Han HY, Wan SQ, Chen B (2014) Six-year fertilization modifies the biodiversity of arbuscular mycorrhizal fungi in a temperate steppe in Inner Mongolia. Soil Biol Biochem 69:371–381
De Beenhouwer M, Van Geel M, Ceulemans T, Muleta D, Lievens B, Honnay O (2015) Changing soil characteristics alter the arbuscular mycorrhizal fungi communities of Arabica coffee (Coffea arabica) in Ethiopia across a management intensity gradient. Soil Biol Biochem. doi:10.1016/j.soilbio.2015.1008.1037
Dormann CF, Gruber B, Fruend J (2008) Introducing the bipartite package: analysing ecological networks. R News 8:8–11
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol Monogr 67:345–366
Edgar R (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998
Gaur A, Adholeya A, Mukerji KG (2000) On-farm production of VAM inoculum and vegetable crops in marginal soil amended with organic matter. Trop Agric 77:21–26
Gosling P, Mead A, Proctor M, Hammond JP, Bending GD (2013) Contrasting arbuscular mycorrhizal communities colonizing different host plants show a similar response to a soil phosphorus concentration gradient. New Phytol. 198:546–556
Gryndler M, Larsen J, Hrselova H, Rezacova V, Gryndlerova H, Kubat J (2006) Organic and mineral fertilization, respectively, increase and decrease the development of external mycelium of arbuscular mycorrhizal fungi in a long-term field experiment. Mycorrhiza 16:159–166
Guo D, Xia M, Wei X, Chang W, Liu Y, Wang Z (2008) Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol. 180:673–683
Hijri I, Sykorova Z, Oehl F, Ineichen K, Mader P, Wiemken A, Redecker D (2006) Communities of arbuscular mycorrhizal fungi in arable soils are not necessarily low in diversity. Mol Ecol 15:2277–2289
Jensen A, Jakobsen I (1980) The occrrence of vesicular-arbuscular mycorrhiza in barley and wheat grown in some Danish soils with different fertilizer treatments. Plant Soil 55:403–414
Johnson NC (1993) Can fertilization of soil select less mutualistic mycorrhizae? Ecol Appl 3:749–757
Johnson N (2010) Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol. 185:631–647
Johnson NC, Angelard C, Sanders IR, Kiers ET (2013) Predicting community and ecosystem outcomes of mycorrhizal responses to global change. Ecol Lett 16:140–153
Jost L (2006) Entropy and diversity. Oikos 113:363–375
Kahiluoto H, Ketoja E, Vestberg M (2000) Promotion of utilization of arbuscular mycorrhiza through reduced P fertilization 1. Bioassays in a growth chamber. Plant Soil 227:191–206
Kunin V, Engelbrektson A, Ochman H, Hugenholtz P (2010) Wrinkles in the rare biosphere: pyrosequencing errors can lead to artificial inflation of diversity estimates. Environ Microbiol 12:118–123
Lin XG, Feng YZ, Zhang HY, Chen RR, Wang JH, Zhang JB, Chu HY (2012) Long-Term Balanced Fertilization Decreases Arbuscular Mycorrhizal Fungal Diversity in an Arable Soil in North China Revealed by 454 Pyrosequencing. Environ. Sci. Technol. 46:5764–5771
Liu Y, Mao L, He X, Cheng G, Ma X, An L, Feng H (2012) Rapid change of AM fungal community in a rain-fed wheat field with short-term plastic film mulching practice. Mycorrhiza 22:31–39
Lumini E, Orgiazzi A, Borriello R, Bonfante P, Bianciotto V (2010) Disclosing arbuscular mycorrhizal fungal biodiversity in soil through a land-use gradient using a pyrosequencing approach. Environ Microbiol 12:2165–2179
Margulies M, Egholm M, Altman W, Attiya S, Bader J, Bemben L, Berka J, Braverman M, Chen Y-J, Chen Z (2005) Genome sequencing in microfabricated high-density picolitre reactors. Nature 437:376–380
McCune B and Mefford M J (2006) PC-ORD, Multivariate Analysis of Ecological Data. MjM Software, Gleneden Beach, OR, home.centurytel.net/~mjm/pcordwin.htm
Miller DD, Domoto PA, Walker C (1985) Mycorrhizal fungi at eighteen apple rootstock plantings in the United States. New Phytol. 100:379–391
Oksanen J, Blanchet G, Kindt R, Legendre P, Minchin P, Simpson G, Solymos P, Stevens M and H W (2013) Vegan: Community Ecology Package. R package version 2.0–10.
Öpik M, Moora M, Liira J, Urmas K, Martin Z, Robin S (2003) Divergent arbuscular mycorrhizal fungal communities colonize roots of Pulsatilla spp in boreal Scots pine forest and grassland soils. New Phytol:160
Öpik M, Metsis M, Daniell TJ, Zobel M, Moora M (2009) Large-scale parallel 454 sequencing reveals host ecological group specificity of arbuscular mycorrhizal fungi in a boreonemoral forest. New Phytol. 184:424–437
Öpik M, Vanatoa A, Vanatoa E, Moora M, Davison J, Kalwij J, Reier U, Zobel M (2010) The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol. 188:223–241
Peng SDM, Eissenstat SDM, Graham JH, Williams K, Hodge NC (1993) Growht depression in mycorrhizal citrus at high phosphorus supply: analysis of carbon costs. Plant Physiol 101:1063–1071
Rillig M, Mummey D (2006) Mycorrhizas and soil structure. New Phytol. 171:41–53
Robertson G, Coleman D, Bledsoe C, Sollins P (1999) Standard soil methods for long-term ecological research. Oxford University Press, New York, USA
Rodriguez-Girones MA, Santamaria L (2006) A new algorithm to calculate the nestedness temperature of presence-absence matrices. J Biogeogr 33:924–935
Sainz MJ, Taboada-Castro MT, Vilarino A (1998) Growth, mineral nutrition and mycorrhizal colonization of red clover and cucumber plants grown in a soil amended with composted urban wastes. Plant Soil 205:85–92
Sanders IR (2004) Plant and arbuscular mycorrhizal fungal diversity - are we looking at the relevant levels of diversity and are we using the right techniques? New Phytol. 164:415–418
Santos J, Finlay R, Tehler A (2006) Molecular analysis of arbuscular mycorrhizal fungi colonising a semi-natural grassland along a fertilisation gradient. New Phytol. 172:159–168
Sato K, Suyama Y, Saito M, Sugawara K (2005) A new primer for discrimination of arbuscular mycorrhizal fungi with polymerase chain reaction-denature gradient gel electrophoresis. Grassl Sci 51:179–181
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Sikes B, Cottenie K, Klironomos J (2009) Plant and fungal identity determines pathogen protection of plant roots by arbuscular mycorrhizas. J Ecol 97:1274–1280
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic Press, Cambridge, UK
Sylvia DM, Schenk NC (1983) Application of superphosphate to mycorrhizal plant stimulates sporulation of phosphorus-tolerant vesicular-arbuscular mycorrhizal fungi. New Phytol. 95:655–661
Tedersoo L, Nilsson R, Abarenkov K, Jairus T, Sadam A, Saar I, Bahram M, Bechem E, Chuyong G, Kõljalg U (2010) 454 Pyrosequencing and Sanger sequencing of tropical mycorrhizal fungi provide similar results but reveal substantial methodological biases. New Phytol. 188:291–301
Van Geel M, Busschaert P, Honnay O, Lievens B (2014) Evaluation of six primer pairs targeting the nuclear rRNA operon for characterization of arbuscular mycorrhizal fungal (AMF) communities using 454 pyrosequencing. J Microbiol Methods 106:93–100
Van Geel M, Ceustermans A, Van Hemelrijck W, Lievens B, Honnay O (2015) Decrease in diversity and changes in community composition of arbuscular mycorrhizal fungi in roots of apple trees with increasing orchard management intensity across a regional scale. Mol Ecol 24:941–952
Verbruggen E, Kiers ET (2010) Evolutionary ecology of mycorrhizal functional diversity in agricultural systems. Evol Appl 3:547–560
Verbruggen E, van der Heijden M, Weedon J, Kowalchuk G, Röling W (2012) Community assembly, species richness and nestedness of arbuscular mycorrhizal fungi in agricultural soils. Mol Ecol 21:2341–2353
Veresoglou S, Rillig M (2012) Suppression of fungal and nematode plant pathogens through arbuscular mycorrhizal fungi. Biol Lett 8:214–217
Waud M, Busschaert P, Ruyters S, Jacquemyn H, Lievens B (2014) Impact of primer choice on characterization of orchid mycorrhizal communities using 454 pyrosequencing. Mol. Ecol. Res. 14:679–699
Werner GD, Kiers ET (2015) Partner selection in the mycorrhizal mutualism. New Phytol. 205:1437–1442
Wilson G, Rice C, Rillig M, Springer A, Hartnett D (2009) Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: results from long-term field experiments. Ecol Lett 12:452–461
Acknowledgments
This study was funded by the project IWT-LA 110775 of the Institute for the Promotion of Innovation by Science and Technology in Flanders.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Tatsuhiro Ezawa.
Electronic supplementary material
Online Resource 1
Rarefaction curves of arbuscular mycorrhizal fungal operational taxonomic units for all treatments. The chemical and slow-release fertilizers are shown in red and green, respectively. Each treatment consisted of four samples (1399 sequences per sample), except the Chemical fertilizer 20 and the Mushroom compost treatment which consisted of only three samples after rarefying. All rarefaction curves tended to saturation. Online Resource 2 List of the 39 operational taxonomic units (OTUs), identified at a 3 % sequence dissimilarity cut-off discovered in our study. The taxonomic affiliations were obtained by BLAST analysis against the MaarjAM database. (PDF 575 kb)
Rights and permissions
About this article
Cite this article
Van Geel, M., De Beenhouwer, M., Ceulemans, T. et al. Application of slow-release phosphorus fertilizers increases arbuscular mycorrhizal fungal diversity in the roots of apple trees. Plant Soil 402, 291–301 (2016). https://doi.org/10.1007/s11104-015-2777-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2777-x