Abstract
Aims
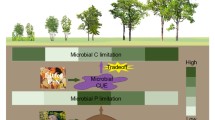
Our aim was to examine the linkage between tree species and anaerobic microbial activity in a forested wetland. If plant species have a unique influence on soil microorganisms, then knowing plant species identity has tremendous potential to predict rates of microbial activity belowground. We expected the linkage to occur via leaf litter decay rate and differences in leaf litter carbon (C) fractions.
Methods
We used leaf litter from 10 tree species (angiosperm and gymnosperm, deciduous and evergreen) and soil from a forested wetland in New York State. We quantified leaf litter decay on the soil surface and correlated variation in rates with leaf mass per area (LMA) and several C fractions. We extracted water-soluble compounds from green leaves and leaf litter and tested their ability to fuel anaerobic carbon dioxide (CO2) production and methane (CH4) production. We also added leaf litter directly to soil to examine how the residue might influence anaerobic microbial activity.
Results
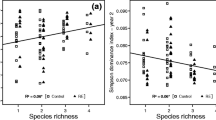
Leaf decay rates exhibited a negative linear relationship with LMA. Variations in rates of soil methane (CH4) production were species specific and greater with gymnosperms (mean = 8.6 μmol g−1 d−1) than angiosperms (mean = 3.7 μmol g−1 d−1). Rates of anaerobic CO2 production were less affected by plant species. Pectin and hemicellulose in leaf litter was particularly conducive to anaerobic decomposition and CH4 production.
Conclusions
The influence of plant species on soil microbial activity will become clearer when we can better quantify leaf C fractions and understand the decomposability of each fraction in relationship to others.



Similar content being viewed by others
References
Anderson JPE, Domsch KH (1978) A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol Biochem 10:215–221
Anderson T-H, Domsch KH (1985) Determination of ecophysiological maintenance carbon requirements of soil microorganisms in a dormant state. Biol Fertil Soils 1:81–89
Augusto L, De Schrijver A, Vesterdal L, Smolander A, Prescott C, Ranger J (2014) Influences of evergreen gymnosperm and deciduous angiosperm tree species on the functioning of temperate and boreal forests. Biol Rev. doi:10.1111/brv.12119
Ayres E, Steltzer H, Berg S, Wallenstein MD, Simmons BL, Wall DH (2009) Tree species traits influence soil physical, chemical, and biological properties in high elevation forests. PLoS One 4(6):e5964
Bache R, Pfennig N (1981) Selective isolation of Acetobacterium woodii on methoxylated aromatic acids and determination of growth yields. Arch Microbiol 130:255–261
Baldrian P, Lindahl B (2011) Decomposition in forest ecosystems: after decades of research still novel findings. Fungal Ecol 4:359–361
Beerling DJ, Berner RA, Mackenzie FT, Harfoot M, Pyle JA (2009) Methane and the CH4-related greenhouse effect over the past 400 million years. Am J Sci 309:97–113
Berg B, Staaf H (1980) Decomposition rate and chemical changes of Scots pine needle litter. II. Influence of chemical composition. Ecol Bull 32:373–390
Bonanomi G, Incerti G, Ciannino F, Mingo A, Lanzotti V, Mazzoleni S (2013) Litter quality assessed by solid state 13C NMR spectroscopy predicts decay rate better than C/N and lignin/N ratios. Soil Biol Biochem 56:40–48
Bräuer SL, Yashiro E, Ueno NG, Yavitt JB, Zinder SH (2006) Characterization of acid-tolerant H2/CO2-utilizing methanogenic enrichment cultures from an acidic peat bog in New York State. FEMS Microbiol Ecol 57:206–216
Cocaign M, Wilberg E, Lindley ND (1991) Sequential demethoxylation reactions during methylotrophic growth of methoxylated aromatic substrates with Eubacterium limosum. Arch Microbiol 155:496–499
Coles JRP, Yavitt JB (2004) Linking belowground carbon allocation to anaerobic CH4 and CO2 production in a forested peatland, New York State. Geomicrobiol J 21:445–454
Cornelissen JHC, Pérez‐Harguindeguy N, Díaz S, Grime JP, Marzano B, Cabido M, Vendramini F, Cerabolini B (1999) Leaf structure and defence control litter decomposition rate across species and life forms in regional floras on two continents. New Phytol 143:191–200
Cornwell WK, Cornelissen JHC, Amatangelo K et al (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6:850–861
De Wit R, Bouvier T (2006) ‘Everything is everywhere, but, the environment selects’; what did Baas Becking and Beijerinck really say? Environ Microbiol 8:755–758
Diefendorf AF, Mueller KE, Wing SL, Koch PL, Freeman KH (2010) Global patterns in leaf 13C discrimination and implications for studies of past and future climate. Proc Natl Acad Sci U S A 107:5738–5743
Don A, Kalbitz K (2005) Amounts and degradability of dissolved organic carbon from foliar litter at different decomposition stages. Soil Biol Biochem 37:2171–2179
Fanin N, Hättenschwiler S, Fromin N (2014) Litter fingerprint on microbial biomass, activity, and community structure in the underlying soil. Plant Soil 379:79–91
Flett RJ, Hamilton RD, Campbell NER (1976) Aquatic acetylene-reduction techniques: solutions to several problems. Can J Microbiol 22:43–51
Franklin RB, Mills AL (2003) Multi‐scale variation in spatial heterogeneity for microbial community structure in an eastern Virginia agricultural field. FEMS Microbiol Ecol 44:335–346
Fry SC (1986) Cross-linking of matrix polymers in the growing cell walls of angiosperms. Annu Rev Plant Physiol 37:165–186
Grayston SJ, Wang S, Campbell CD, Edwards AC (1998) Selective influence of plant species on microbial diversity in the rhizosphere. Soil Biol Biochem 30:369–378
Greb SF, DiMichele WA, Gastaldo RA (2006) Evolution and importance of wetlands in Earth history. In: Greb S, DiMichele WA (eds) Wetlands through time, vol 399, Geological Society of America Special Paper., pp 1–40
Harrington GJ, Eberle J, Le-Page BA, Dawson M, Hutchison JH (2012) Arctic plant diversity in the Early Eocene greenhouse. Proc R Soc B 279:1515–1521
Hättenschwiler S, Aeschlimann B, Coûteaux MM, Roy J, Bonal D (2008) High variation in foliage and leaf litter chemistry among 45 tree species of a neotropical rainforest community. New Phytol 179:165–175
Hongve D, Van Hees PAW, Lundström US (2000) Dissolved components in precipitation water percolated through forest litter. Eur J Soil Sci 51:667–677
Jung H-JG (1997) Analysis of forage fiber and cell walls in ruminant nutrition. J Nutr 127:810S–813S
Kalbitz K, Schmerwitz J, Schwesig D, Matzner E (2003) Biodegradation of soil-derived dissolved organic matter as related to its properties. Geoderma 113:273–291
Keiser AD, Knoepp JD, Bradford MA (2013) Microbial communities may modify how litter quality affects potential decomposition rates as tree species migrate. Plant Soil 372:167–176
Kiikkilä O, Kanerva S, Kitunen V, Smolander A (2014) Soil microbial activity in relation to dissolved organic matter properties under different tree species. Plant Soil 377:169–177
King JS, Pregitzer KS, Zak DR, Kubiske ME, Holmes WE (2001) Correlation of foliage and litter chemistry of sugar maple, Acer saccharum, as affected by elevated CO2 and varying N availability, and effects on decomposition. Oikos 94:403–416
Klotzbücher T, Kaiser K, Guggenberger G, Gatzek C, Kalbitz K (2011) A new conceptual model for the fate of lignin in decomposing plant litter. Ecology 92:1052–1062
LePage BA, Yang H, Matsumoto M (2005) The evolution and biogeographic history of Metasequoia. In: LePage BA, Williams CJ, Yang H (eds) The geobiology and ecology of Metasequoia. Springer, New York, pp 4–81
MacDonald GM, Beilman DW, Kremenetski KV, Sheng YW, Smith LC, Velichko AA (2006) Rapid early development of circumarctic peatlands and atmospheric CH4 and CO2 variations. Science 314:285–288
Matthews E, Fung I (1987) Methane emissions from natural wetlands: global distribution, area, and environmental characteristics of sources. Glob Biogeochem Cycles 1:61–86
McIver EE, Basinger JF (1999) Early tertiary floral evolution in the Arctic. Ann Mo Bot Gard 86:523–545
McLeod AR, Newsham KK, Fry SC (2007) Elevated UV-B radiation modifies the extractability of carbohydrates from leaf litter of Quercus robur. Soil Biol Biochem 39:116–126
McLeod AR, Fry SC, Loake GJ, Messenger DJ, Reay DS, Smith KA, Yun BW (2008) Ultraviolet radiation drives methane emissions from terrestrial plant pectins. New Phytol 180:124–132
Meier CL, Bowman WD (2008) Links between plant litter chemistry, species diversity, and below-ground ecosystem function. Proc Natl Acad Sci U S A 105:19780–19785
Michalzik B, Kalbitz K, Park J-H, Solinger S, Matzner E (2001) Fluxes and concentrations of dissolved organic carbon and nitrogen - a synthesis for temperate forests. Biogeochemistry 52:173–205
Miller DN, Ghiorse WC, Yavitt JB (1999) Seasonal patterns and controls on methane and carbon dioxide fluxes in forested swamp pools. Geomicrobiol J 16:325–331
Miller DN, Yavitt JB, Madsen EL, Ghiorse WC (2004) Methanotrophic activity, abundance, and diversity in forest swamp pools: spatiotemporal dynamics and influences on methane fluxes. Geomicrobiol J 21:257–271
Miyajima T, Wada E, Hanba YT, Vijarnsorn P (1997) Anaerobic mineralization of indigenous organic matters and methanogenesis in tropical wetland soils. Geochcim Cosmochim Acta 61:3739–3751
Nemergut DR, Cleveland CC, Wieder WR, Washenberger C, Townsend AR (2010) Plot-scale manipulations of organic matter inputs to soils correlate with shifts in microbial community composition in a lowland tropical rain forest. Soil Biol Biochem 42:2153–2160
Nykvist N (1963) Leaching and decomposition of water-soluble organic substances from different types of leaf and needle litter. Stud For Suec 3:3–31
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–331
O’Regan M, Williams C, Frey K, Jakobsson M (2011) A synthesis of the long-term paleoclimatic evolution of the Arctic. Oceanography 24:66–80
Orwin KH, Wardle DA, Greenfield LG (2006) Ecological consequences of carbon substrate identity and diversity in a laboratory study. Ecology 87:580–593
Orwin KH, Buckland SM, Johnson D, Turner BL, Smart S, Oakley S, Bardgett RD (2010) Linkages of plant traits to soil properties and the functioning of temperate grassland. J Ecol 98:1074–1083
Peichl M, Moore TR, Arain MA, Dalva M, Brodkey D, McLaren J (2007) Concentrations and fluxes of dissolved organic carbon in an age-sequence of white pine forests in Southern Ontario, Canada. Biogeochemistry 86:1–17
Podobina VM (2003) Paleocene biota of the West Siberian plain. In: Wing SL, Gingerich PD, Schmitz B, Thomas E (eds) Causes and consequences of globally warm climate in the Early Paleogene, vol 369, The Geological Society of America Special Paper., pp 181–204
Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R (2009) Causes and consequences of variation in leaf mass per area (LMA): a meta‐analysis. New Phytol 182:565–588
Royer DL, Sack L, Wilf P et al (2007) Fossil leaf economics quantified: calibration, Eocene case study, and implications. Paleobiology 33:574–589
Schadel C, Blochl A, Richter A, Hoch G (2010) Quantification and monosaccharide composition of hemicelluloses from different plant functional types. Plant Physiol Biochem 48:1–8
Schink B, Zeikus JG (1982) Microbial ecology of pectin decomposition in anoxic lake sediments. J Gen Microbiol 128:393–404
Schreeg LA, Mack MC, Turner BL (2013) Nutrient-specific solubility patterns of leaf litter across 41 lowland tropical woody species. Ecology 94:94–105
Šnajdr J, Cajthaml T, Valášková V, Merhautová V, Petránková M, Spetz P, Leppänen K, Baldrian P (2011) Transformation of Quercus petraea litter: successive changes in litter chemistry are reflected in differential enzyme activity and changes in the microbial community composition. FEMS Microbiol Ecol 75:291–303
Steinmann P, Huon S, Roos-Barraclough F, Föllmi KA (2006) A peat core based estimate of late-glacial and Holocene methane emissions from northern peatlands. Glob Planet Chang 53:233–239
Ström L, Ekberg A, Mastepanov M, Christensen TR (2003) The effect of vascular plants on carbon turnover and methane emissions from a tundra wetland. Glob Chang Biol 9:1185–1192
Sutton-Grier AE, Megonigal JP (2011) Plant species traits regulate methane production in freshwater wetland soils. Soil Biol Biochem 43:412–420
Swan CM, Palmer MA (2004) Leaf diversity alters litter breakdown in a Piedmont stream. J N Am Benthol Soc 23:15–28
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in terrestrial ecosystems. Studies in ecology, vol 5. Blackwell, Oxford
Thevenot M, Dignac M-F, Rumpel C (2010) Fate of lignins in soils: a review. Soil Biol Biochem 42:1200–1211
Van Soest PJ (1994) Nutritional ecology of the ruminant, 2nd edn. Cornell University Press, Ithaca
Weishaar JL, Aiken GR, Bergamaschi BA, Fram MS, Fujii R, Mopper K (2003) Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ Sci Technol 37:4702–4708
Wilhelm E, Battino R, Wilcock RJ (1977) Low-pressure solubility of gases in liquid water. Chem Rev 77:219–262
Williams CJ, Yavitt JB (2003) Botanical composition of peat and degree of peat decomposition in three temperate peatlands. Ecoscience 10:85–95
Williams CJ, Yavitt JB (2010) Temperate wetland methanogenesis: the importance of vegetation type and root ethanol production. Soil Sci Soc Am J 74:317–325
Williams CJ, Johnson AH, LePage BA, Vann DR, Sweda T (2003) Reconstruction of tertiary metasequoia forests II. Structure, biomass and productivity of Eocene floodplain forests in the Canadian arctic. Paleobiology 29:271–292
Wright IJ, Reich PB, Westoby M et al (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Yavitt JB, Fahey TJ (1986) Long-term litter decay and forest floor leaching in Pinus contorta ecosystems, southeastern Wyoming. J Ecol 74:525–545
Yavitt JB, Williams CJ, Wieder RK (1997) Production of methane and carbon dioxide in peatland ecosystems across North America: effects of temperature, aeration and organic chemistry of peat. Geomicrobiol J 14:299–316
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Paul Bodelier.
Rights and permissions
About this article
Cite this article
Yavitt, J.B., Williams, C.J. Linking tree species identity to anaerobic microbial activity in a forested wetland soil via leaf litter decomposition and leaf carbon fractions. Plant Soil 390, 293–305 (2015). https://doi.org/10.1007/s11104-015-2403-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2403-y