Abstract
The plant-specific homeodomain-leucine zipper I subfamily is involved in the regulation of various biological processes, particularly growth, development and stress response. In the present study, we characterized four BnaHB6 homologues from Brassica napus. All BnaHB6 proteins have transcriptional activation activity. Structural and functional data indicate the complex role of BnaHB6 genes in regulating biological processes, with some functions conserved and others diverged. Transcriptional analyzes revealed that they are induced in a similar manner in different tissues but show different expression patterns in response to stress and circadian rhythm. Only the BnaA09HB6 and BnaC08HB6 genes are expressed under dehydration and salt stress, and in darkness. The partial transcriptional overlap of BnaHB6s with the evolutionarily related genes BnaHB5 and BnaHB16 was also observed. Transgenic Arabidopsis thaliana plants expressing a single proBnaHB6::GUS partially confirmed the expression results. Bioinformatic analysis allowed the identification of TF-binding sites in the BnaHB6 promoters that may control their expression under stress and circadian rhythm. ChIP-qPCR analysis revealed that BnaA09HB6 and BnaC08HB6 bind directly to the promoters of the target genes BnaABF4 and BnaDREB2A. Comparison of their expression patterns in the WT plants and the bnac08hb6 mutant showed that BnaC08HB6 positively regulates the expression of the BnaABF4 and BnaDREB2A genes under dehydration and salt stress. We conclude that four BnaHB6 homologues have distinct functions in response to stress despite high sequence similarity, possibly indicating different binding preferences with BnaABF4 and BnaDREB2A. We hypothesize that BnaC08HB6 and BnaA09HB6 function in a complex regulatory network under stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
BnaHB6 homologues show different response to abiotic stress and light conditions and differential transcriptional regulation of BnaABF4 and BnaDREB2A target genes under dehydration and salt stress.
Introduction
The regulation of gene transcription is a highly complex process in which transcription factors (TFs) act as regulatory proteins to activate or repress transcription by binding to specific DNA sequences in promoters of target genes. In Arabidopsis thaliana, about 40% of the identified TFs, including HD-Zip, NAC, AP2/EREBP, GARP, ARF, SBP, MADS-box, ABI3/VP1 and WRKY, are plant-specific genes.
Plant-specific homeodomain-leucine zipper (HD-Zip) TFs are important regulators of growth and development as well as response to stress that control various signaling pathways through interactions with other genes (Ariel et al. 2007). A highly conserved 60-amino acid homeodomain (HD) and the adjacent less conserved leucine zipper motif (LZ) play an important role in this process (Henriksson et al. 2005). The HD-Zip gene family is divided into four subfamilies: HD-Zip I, HD-Zip II, HD-Zip III and HD-Zip IV, which differ in their DNA binding specificity, gene structures and biological functions (Henriksson et al. 2005; Ariel et al. 2007). Among them, HD-Zip I genes are involved in developmental processes such as root and stem elongation, leaf morphology determination, flowering and pollen hydration, and stress response (Wang et al. 2003; Henriksson et al. 2005; Ariel et al. 2007; Ré et al. 2014; Ribone et al. 2015). In general, the members of the HD-Zip I subfamily show structural similarity and functional redundancy (Sessa et al. 1993; Palena et al. 1999; Johannesson et al. 2001; Żyła and Babula-Skowrońska 2023, for review). However, more detailed molecular studies have revealed their functional diversity in the regulation of biological processes, as evidenced by altered expression patterns in response to external stimuli, divergence in binding sites, differences in interaction with other proteins and the specificity of their site activity (in organs or tissues) (Henriksson et al. 2005; Ré et al. 2014; Capella et al. 2015; Perotti et al. 2019, 2021). One of the mechanisms responsible for this are changes within the LZ of certain HD-Zip I proteins, which can influence the efficiency and specificity of the DNA-binding process. It is also hypothesized that changes in regulatory motifs outside of HD and LZ involved in protein-protein interactions may be a source of functional diversity between evolutionarily related HD-Zip I genes (Olsson et al. 2004; Arce et al. 2011; Capella et al. 2014; Ré et al. 2014; Ribone et al. 2015). For example, the paralogous genes AtHB7 and AtHB12 in A. thaliana are upregulated under drought stress and in response to ABA, but they show specific, non-overlapping functions in root development; only AtHB7 is involved in root growth at early stages of plant development (Olsson et al. 2004; Ré et al. 2014). In contrast to AtHB7 and AtHB12, AtHB6 has been described as a negative regulator of the ABA signaling pathway that physically interacts with ABI1 (serine/threonine protein phosphatase 2C) to act downstream under drought stress (Söderman et al. 1999; Himmelbach et al. 2002; Henriksson et al. 2005).
Many orthologs of ATHB genes from A. thaliana have been found in other plant species such as sunflower, tomato, cucumber, cotton, rice and Brassica (Lin et al. 2008; Manavella et al. 2008; Ariel et al. 2010; Liu et al. 2013; Zhang et al. 2016; Yang et al. 2020; Peng et al. 2022). Despite their evolutionary relationship, they show only partial functional similarity at the transcriptional level. Many of them have evolved novel tissue preferences that have led to changes in morphological traits to facilitate adaptation to different environmental conditions.
Oilseed rape (Brassica napus L.) is an allopolyploid species whose genomes have evolved by whole-genome triplication (Parkin et al. 2005). Many genes in these plants are preferentially maintained in a duplicated state with very similar sequences, but they have gradually changed their functions by sub- or neofunctionalization (Chalhoub et al. 2014; Babula-Skowrońska et al. 2015; Jiang et al. 2015). Recently, the expansion and expression patterns of HD-Zip genes in the Brassica species have been documented under different abiotic stress conditions (Jing et al. 2017; Khan et al. 2018; Peng et al. 2022; Yin et al. 2023). These studies showed dynamic changes in the expression patterns of homeobox genes in different tissues in relation to their response to abiotic stress and in the distribution of functional domains. So far, only a few HD-Zip I genes have been identified in oilseed rape (Brassica napus L.), so their functions are still poorly known (Peng et al. 2022). Previous studies have shown that AtHB6 is involved in stress response processes in A. thaliana (Himmelbach et al. 2002; Henriksson et al. 2005). However, its role, particularly in the context of duplication, has not yet been described in the Brassica genus.
In this study, we characterized four BnaHB6 homologues in B. napus in terms of their phylogenetic relationship, structure, transcriptional activity and expression patterns. Based on the functional differences in induction under dehydration and salt stress, we identified factors contributing to their retention in the genome. We found that only two of the four BnaHB6 genes, namely BnaA09HB6 and BnaC08HB6 are strongly induced under dehydration and salt stress. Our studies have also revealed a novel antagonistic function of the gene pairs BnaA09HB6/BnaC08HB6 and BnaA04HB6/BnaC04HB6 under light conditions. Furthermore, we found that the evolutionarily related genes BnaHB6, BnaHB5 and BnaHB16 have partially overlapping functions in plant development, response to ABA and stress response. ChIP-qPCR analyzes and transcriptional profiling of BnaHB6s in WT plants and the bnac08hb6 mutant revealed the role of BnaA09HB6 and BnaC08HB6 in regulating the expression of four BnaABF4 and two BnaDREB2A homologues in response to stress through direct binding to their promoter regions. We conclude that BnaA09HB6 and BnaC08HB6 are transcriptional activators of some BnaABF4 and BnaDREB2A genes under dehydration and salt stress.
Materials and methods
Plant material, growth conditions and stress treatments
B. napus cv Monolit (Plant Breeding Strzelce Ltd., Co.) and A. thaliana Heyhn ecotype Columbia (Col-0) were used in this study. Line G2000-375-1 from the B. napus TILLING population with point mutations in the BnaC08HB6 gene was kindly provided by Prof. Ian Bancroft (University of York, United Kingdom). All plants were grown on soil or Hoagland medium in a controlled growth chamber at 20/18 °C under long-day photoperiod (16-h/8-h light/dark cycle). Whole plants were harvested from the first to the fourth week of growth including cotyledons or true leaves, hypocotyl and roots for analysis and at fixed daily time points. Stress conditions were performed according to Babula-Skowrońska (2021) with minor modifications. For dehydration and salt stress, three-week-old oilseed rape plants were transferred to Hoagland medium containing 15% PEG8000 and 200 mM NaCl, respectively. For ABA treatment, three-week-old B. napus seedlings were transferred to Hoagland medium containing 50 µM ± ABA. For cold stress, three-week-old plants were placed at + 4 °C for 7 days. Leaves and roots were harvested 24 h or 7 days after treatment and then immediately frozen in liquid nitrogen. Circadian rhythm was analyzed in a 24-h cycle with 16 h of light and 8 h of darkness and samples were collected every two hours, from one hour after lights on (9:00 am) on day 0 to one hour before lights on (7:00 am) on day 1. All experiments were performed in three independent biological replicates.
Isolation and cloning of the BnaHB6, BnaHB5 and BnaHB16 genes
Total DNA and RNA were isolated from B. napus leaves using the DNeasy Plant Kit and the RNeasy Plant Mini Kit (Qiagen), respectively according to the manufacturer’s instructions. The genomic and full-length coding sequences of the BnaHB6, BnaHB5 and BnaHB16 genes were isolated using DNA and cDNA as templates (Table S1). The sequences obtained were cloned into the pENTR/D-TOPO vector and then subcloned into compatible vectors using the Gateway system. The primers used are listed in Supplementary Table S2. The B. napus genes are named according to the gene nomenclature system for the Brassica genus, which specifies the species-genome-gene name order (Ostergaard and King 2008).
Phylogenetic tree reconstruction and bioinformatic analyses
The predicted amino acid sequences of HD-Zip I proteins of A. thaliana and B. napus were used to reconstruct the phylogenetic trees. First, these sequences were aligned using the Clustal Omega program and then the phylogenetic trees were reconstructed using the neighbor-joining algorithm implemented in MEGA11 (https://megasoftware.net/). A total of 1,000 bootstrap replicates were used to calculate the confidence level for each node. Motif and domain analyzes of BnaHB6 proteins were performed using the SMART program (http://smart.embl.de/smart/set_mode.cgi?NORMAL=1). Exon-intron analysis of BnaHB6 genes was performed by aligning of the genomic and CDS sequences and then visualized using the GSDS gene structure display server (http://gsds.gao-lab.org/). Putative ABA-, phytohormone-, light- and stress-responsive cis-elements in approximately 1.5-kb promoter regions of BnaHB6 genes were identified using the PlantCare database (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
Transcriptional activation assay in yeast cells
To study transcriptional activity, the coding regions of BnaHB6s were cloned separately into the pGBKT7 vector carrying the GAL4-responsive GAL1 promoter and the HIS3 reporter gene and then independently introduced into yeast strain AH109 using the Yeastmaker™ Yeast Transformation System 2 (TaKaRa). The empty pGBKT7 vector was used as a negative control. The transformed yeasts with four BnaHB6 constructs were spotted on SD/-Trp and SD/-Trp/-Ade/-His/X-α-gal media and grown at 30 °C for 3 days to test the transcriptional activity. For the quantitative assay, β-galactosidase activity was monitored based on O-Nitrophenyl β-D-galactoside (ONPG) as a substrate in a liquid assay according to the manufacturer’s instructions (Clontech). The primers used are listed in Supplementary Table S2.
Isolation and cloning of the BnaHB6 promoter regions
Over 1.5-kb upstream of the start codon of the BnaHB6 genes were isolated from B. napus genomic DNA and cloned separately into the pBract104-GUS vector (BRACT, John Innes Centre, UK) and verified by sequencing (proBnaHB6::GUS). The recombinant vectors were individually introduced into Agrobacterium tumefaciens strain AGL-1 by electroporation and subsequently transformed into wild-type plants of A. thaliana (Col-0) by the floral dip method (Clough and Bent 1998). The putative transformants were selected by placing the seeds on agar-solidified ½ MS medium containing 50 mg l−1 kanamycin and the integrity of the transgenes was determined by PCR with specific oligonucleotides for each construct. Five to ten positive independent lines of T3 generation for each construct were used for the analyses. For dehydration and salt stress, two-week-old transgenic A. thaliana seedlings were placed on medium containing 15% PEG8000 and 200 mM NaCl, respectively for 12 h. For ABA treatment, two-week-old transgenic A. thaliana seedlings were placed on medium containing 50 µM ± ABA for 6 h. The untreated transgenic plants were used as controls. All primer sequences for vector construction are listed in Supplementary Table S2.
Histochemical GUS staining
Histochemical GUS staining for the GUS activity assay in the transgenic A. thaliana plants was performed as described previously (Babula-Skowrońska et al. 2015). Whole seedlings or different tissues were immersed in the staining buffer containing 1 mM 5-bromo-4-chloro-3-inodyl-β-glucuronic acid (X-Gluc), 100 mM sodium phosphate (pH 7.0), 0.1 mM EDTA, 0.5 mM ferricyanide and 0.1% Triton X-100 at 37 °C for 6 h. The GUS-stained seedlings were washed in 70% ethanol. Three to five independent biological replicates were tested, each with three technical replicates.
Isolation of total RNA, cDNA synthesis and quantification of gene expression by qRT-PCR
For gene expression, total RNA isolation and first-strand cDNA synthesis were performed as previously described (Babula-Skowrońska 2021). The qRT-PCR was carried out using the SYBR qPCR Mix (Promega) on the CFX96 real-time system (Bio-Rad) according to the manufacturer’s protocol. Reactions were performed according to the following program: 10 min at 95 °C, followed by 40 cycles at 95 °C for 15 s, 60 °C for 1 min and 72 °C for 1 min. Each experiment was performed with three biological replicates, each containing three technical replicates. Relative expression values were calculated using the 2−ΔΔCt method with PP2A and TIP41 selected as endogenous reference genes by Chen and coworkers (2010). Significance was determined using Student’s t-test. The gene-specific primer sequences used in this work are listed in Supplementary Table S2.
ChIP-qPCR analysis
The CDS of the BnaHB6 genes were cloned into the binary plant vector pEarlyGate103-His under the control of the CaMV35S promoter to obtain constructs for ChIP-qPCR analysis. The 35S::BnaHB6-His constructs were transiently transformed into B. napus leaves according to the protocol of Mooney and Graciet (2020). Genomic DNA was isolated and cross-linked with 1% formaldehyde, quenched with 134 mM glycine and frozen at - 80 °C. Chromatin was disrupted into 0.2-0.5 kb fragments by sonication. Immunoprecipitation followed by quantitative PCR (qPCR) was performed using commercially available anti-His (Cell Signaling Technology). The 10% of the supernatant before the addition of the antibody was used as DNA input control for the qPCR reaction. The enrichment of the BnaABF4 and BnaDREB2A promoters by BnaA09HB6 and BnaC08HB6 was calculated by comparing the DNA immunoprecipitated with the anti-His antibody to the input DNA. The primers are listed in Supplementary Table S2.
Results
Phylogeny and characteristics of the BnaHB6 homologues
Based on syntenic gene analysis between A. thaliana and B. napus (http://brassicadb.cn/#/syntenic-gene/), we identified and cloned four BnaHB6 homologues (BnaA04HB6, BnaC04HB6, BnaA09HB6 and BnaC08HB6) from the B. napus genome. To investigate their evolutionary relationship, we constructed an unrooted phylogenetic tree encompassing all members of the HD-Zip I subfamily of A. thaliana and B. napus and confirmed the duplication events in the Brassica lineage that occurred during the evolution of Brassicaceae (Fig. 1A and 1B). In agreement with previous studies, the HD-Zip I proteins of A. thaliana are clustered into eight clades, with the AtHB6, AtHB5 and AtHB16 proteins located in a common clade β2 (Fig. 1A). Similar to Arabidopsis, the BnaHB6 proteins are also grouped with the BnaHB5 and BnaHB16 proteins in the same clade, confirming their close evolutionary relationship (Fig. 1B). BnaA09HB6 and BnaC08HB6 belong to the same cluster as AtHB6, while BnaA04HB6 and BnaC04HB6 are the more distant their paralogs, suggesting the Brassica lineage-specific whole genome duplication. These results were also confirmed by sequence analyses. The open reading frame (ORF) length of the BnaHB6 genes ranges from 903 bp for BnaA04HB6 and BnaC04HB6 to 936 bp for BnaA09HB6 and BnaC08HB6, and they encode polypeptides between 300 and 311 amino acids. The sequence similarity of these genes ranges from 77.8% between BnaA04HB6 and BnaA09HB6 at the genomic level to 96.8% between BnaA04HB6 and BnaC04HB6 at the amino acid level (Fig. S1A). All BnaHB6 genes have a similar structure with three exons and two introns (Fig. S1B). Motif analysis of the BnaHB6 proteins confirmed the presence of the highly conserved HD and LZ as well as the putative phosphorylation sites (Ser, Thr and Tyr) (Fig. S1C). However, the N- and C-terminal regions of these proteins are more divergent in sequence and have a different arrangement of additional regulatory motifs previously reported in Arabidopsis AtHB6 (Arce et al. 2011; Fig. S1C and S1D). We found similar motif compositions in AtHB6, BnaA09HB6 and BnaC08HB6, suggesting their functional similarity (Fig. S1C). In contrast, only one and two motifs were conserved in BnaA04HB6 and BnaC04HB6, respectively. These differences are due to a single-nucleotide polymorphism and could influence their functional specificity.
Phylogenetic analysis of the HD-Zip I proteins of A. thaliana and B. napus. Phylogenetic trees were constructed using the Neighbor-Joining method in MEGA11. Bootstrap values for 1000 replicates are given for each branch. A. The phylogenetic tree of the 17 HD-Zip I proteins of A. thaliana. The clades are labelled α, β1, β2, γ, δ, ε, φ1 and φ2 according to Henriksson et al. (2005). B. The phylogenetic tree of the HD-Zip I proteins of A. thaliana and B. napus
BnaHB6 act as transcriptional activators
To investigate the transcriptional activity of BnaHB6s, their full-length coding sequences were fused to the GAL4 DNA-binding domain (BD) and then transformed into yeast strain AH109. The BnaHB6 transformants were tested for their ability to activate GAL4 transcription and promote yeast growth in the selective media. They grew well on SD/-Trp medium and showed α-galactosidase activity on SD/-Trp-His-Ade, except for the pGBKT7 empty vector (Fig. 2A). These results were further confirmed by β-galactosidase activity assay (Fig. 2B). This indicates that BnaHB6 are transcriptional activators in yeast and are therefore likely capable of regulating downstream gene expression as TFs.
Transcriptional activity of the BnaHB6 proteins in yeast cells. The full length coding sequences of BnaHB6s were fused to the GAL4 DNA-BD and transformed into yeast strain AH109. A. Transformants were plated out on SD/-Trp (control medium) and SD/-Trp/-Ade-/-His/X-α-Gal (selection medium). Yeast cells expressing GAL4DBD were used as negative control; B. Quantitative β-galactosidase assay with ONPG as substrate. The β-Gal activity was determined as Miller units. The error bars represent three technical replicates
Expression profiling of BnaHB6, BnaHB5 and BnaHB16 genes
In general, evolutionarily related genes may have similar functions. To understand the functional relationship between the four BnaHB6 homologues, we analyzed their expression patterns in different tissues, circadian rhythm, at early developmental stages and under dehydration, salt and cold stresses, and exogenous ABA treatment (Fig. 3). The BnaHB6 genes are expressed in all tissues examined, with high expression in inflorescences and much lower in roots than in other tissues (Fig. 3A). Moreover, the expression of BnaHB6 genes increases rapidly during the first two weeks of plant growth and there are no significant differences in transcript levels between them (Fig. 3A). This indicates that they are functionally redundant in plant growth and development. In contrast, these four BnaHB6 genes show different expression patterns under dehydration and salt stress (Fig. 3B). Two of them, namely BnaA09HB6 and BnaC08HB6, are strongly induced in both leaves and roots under these stress conditions. In addition, the expression of BnaA04HB6 increases in roots in response to dehydration. In contrast, the expression of all BnaHB6 genes is not significantly altered under cold conditions, but BnaA09HB6 and BnaC08HB6 show slightly increased expression. All BnaHB6 genes are induced under exogenous ABA treatment, but the genes regulated by stress conditions show higher expression (Fig. 3B). These results suggest that the orthologous genes BnaA09HB6 and BnaC08HB6 exhibit functional redundancy in the ABA-dependent signaling pathway under dehydration and salt stress. Interestingly, the gene pairs BnaA04HB6/BnaC04HB6 and BnaA09HB6/BnaC08HB6 show opposite fluctuations in expression under light conditions (Fig. 3C). We found that the expression of the BnaA09HB6 and BnaC08HB6 genes generally does not change during light exposure except between 11 and 15 h. However, they show increased expression with a maximum in the fifth hour of darkness.
Expression profiles of the BnaHB6 homologues and their closely related BnaHB5 and BnaHB16 genes at early developmental stages, in different tissues of B. napus plants and in response to abiotic stresses and light conditions. A. RT-qPCR analysis of BnaHB6, BnaHB5 and BnaHB16 transcripts at early developmental stages and in different tissues. Whole plants from the first to fourth week of growth with cotyledons or true leaves, hypocotyl and roots were harvested for analysis; C-cotyledons, L-leaf, S-stem, R-root, I-inflorescence, FB-flower buds and F-flowers. Values are relative to the expression level of the PP2A and TIP41 reference genes; B. RT-qPCR analysis of BnaHB6, BnaHB5 and BnaHB16 transcripts in leaves and roots under different abiotic stresses and ABA treatment. The expression patterns of these genes were determined after NaCl (200 mM), dehydration (15% PEG8000), cold stress (+ 4 °C) and exogenous ABA treatment (50 µM ± ABA); C. RT-qPCR analysis of the transcript of BnaHB6, BnaHB5 and BnaHB16 in a 24-h cycle with 16 h of light and 8 h of darkness. Arabic numerals with L or D below each line indicate the duration of light or dark exposure in hours. The error bars show the standard deviation (SD) for three replicates (n = 3). Asterisks indicate statistically significant differences compared to the untreated control, calculated with the Student’s t-test (*P < 0.005; **P < 0.001)
As the phylogenetic analysis of HD-Zip I members confirmed the close relationship between the BnaHB5, BnaHB6 and BnaHB16 proteins, which may indicate their functional redundancy, we compared their expression profiles in different tissues, early developmental stages, stress conditions and circadian rhythm (Fig. 3). The BnaHB5 and BnaHB16 genes show partially different expression patterns compared to the BnaHB6 genes under the investigated conditions. In particular, the BnaHB5 and BnaHB16 are highly expressed in roots and under cold stress, in contrast to the BnaHB6 genes. However, we found that the expression profiles of BnaA09HB6 and BnaC08HB6 correlate with those of BnaA01HB16, BnaC01HB16, BnaA06HB5 and BnaC0XHB5 under dehydration conditions (Fig. 3B). The different expression patterns of the BnaHB6, BnaHB5 and BnaHB16 genes are observed in the circadian rhythm (Fig. 3C). Interestingly, the orthologous genes BnaA08HB16 and BnaC03HB16 show opposite induction in response to light conditions. BnaA08HB16 is induced in light and its expression is similar to that of BnaC04HB6 but is accelerated by two hours (Fig. 3C). In contrast, BnaC03HB16 is induced in the dark and under dehydration, similar to the orthologous genes BnaA09HB6 and BnaC08HB6, but its maximal expression is delayed by two hours.
Analysis of cis-acting regulatory elements in the promoters of the BnaHB6 genes and GUS activity of BnaHB6 in the A. thaliana background
To understand the mechanisms responsible for the differences in expression patterns of the BnaHB6 homologues under stress and light conditions, we cloned 1.5-kb upstream promoter regions of all BnaHB6 genes and then analyzed them for the location of cis-acting elements associated with response to stress, phytohormones and light-relevant factors using the PlantCARE database. These included the well-characterized elements: the ABA-responsive element (ABRE), MYB binding sites (MYB), auxin response factor, circadian-regulated element (Ebox), light-regulated element (Ibox), AHBP, ethylene-responsive element, circadian, low temperature-responsive element, defense and stress-responsive element (TC element), gibberellin-responsive element, C-repeat/dehydration-responsive element and TAAAG (Fig. 4). We found that stress-responsive cis-elements were more abundant in the promoters of the BnaA09HB6 and BnaC08HB6 genes than in the BnaA04HB6 and BnaC04HB6 genes, consistent with their expression patterns under stress conditions (Figs. 3 and 4). The arrangement of stress-response elements is conserved in the promoters of AtHB6, BnaA09HB6 and BnaC08HB6 confirming their close evolutionary relationship and similar expression patterns under stress conditions. To confirm the transcriptomic studies, we generated transgenic A. thaliana plants expressing the β-glucuronidase (uidA) reporter gene (GUS) under the control of the respective BnaHB6 promoters (proBnaHB6::GUS). We observed differences in the GUS activity of BnaHB6s at early developmental stages and in the tissues examined, with the activity of the BnaC08HB6 promoter being generally higher (Fig. 5A and 5B). The BnaA09HB6 and BnaC08HB6 promoters are expressed at all early developmental stages, whereas the BnaA04HB6 promoter shows no expression in one- and two-week-old seedlings. BnaC08HB6 is preferentially expressed in siliques, but the GUS signal is not detected in flowers, siliques and roots in proBnaA04HB6::GUS plants. The specific expression patterns for individual BnaHB6 are observed in the root section. The BnaA09HB6 and BnaC08HB6 promoters show significant GUS activity in the columella root cap and root core including the xylem, while the BnaC08HB6 promoter is additionally expressed in the endodermis and cortex. Subsequently, these transgenic plants were subjected to NaCl, PEG and ABA treatments to investigate their effects on the BnaHB6 promoter activities (Fig. 5C). In proBnaA09HB6::GUS and proBnaC08HB6::GUS plants, induction of GUS was found in response to dehydration and salt stress. All BnaHB6 plants showed GUS activity after treatment with ABA. These results confirm the expression patterns observed under stress conditions and only partially at different developmental stages and on different organs by qRT-PCR (Fig. 3).
Comparison of the positions of putative transcription factor-binding sites in over 1.5 kb upstream of the transcription start site of AtHB6 and four B. napus BnaHB6 genes. The positions of the binding sites are indicated. The legend with symbols representing potential transcription binding motifs is on the right. The dashed lines connect the cis-acting elements located at conserved positions in the promoters of the AtHB6 and four BnaHB6 genes
GUS staining and activity analysis of proBnaHB6::GUS in transgenic A. thaliana plants: A. in early developmental stages; B. in various tissues such as leaves, flowers, siliques, roots and also in root cells; C. in two-week-old transgenic A. thaliana plants under dehydration, salt stress and ABA treatment. The expression of the BnaHB6 genes was determined by histochemical GUS staining. Seedlings were placed in medium containing 15% PEG8000 and 200 mM NaCl for 12 h for dehydration and salt stress, respectively. For ABA treatment, two-week-old transgenic A. thaliana seedlings were placed in a medium containing 50 µM ± ABA for 6 h
Taken together, these results suggest that the BnaHB6 TFs of B. napus are involved in different ways in the transcriptional control of developmental processes and stress responses.
The role of the BnaA09HB6 and BnaC08HB6 orthologs in regulating the expression of the selected BnaABF4 and BnaDREB2A homologues under abiotic stress conditions
To gain insight into the role of BnaHB6 genes in transcriptional regulation of genes under stress, we investigated their interactions with other stress-induced genes. Since the expression patterns of AtHB6, BnaA09HB6 and BnaC08HB6 are similar under stress, we hypothesized that their role in the expression regulation of target genes is evolutionarily conserved. First, we selected a group of A. thaliana genes involved in drought and salt stress response and controlled by AtHB6 TF from the data collected in PlantPAN 3.0 (http://plantpan.itps.ncku.edu.tw/get_promoter_analysis.php). Among the experimentally verified genes involved in osmotic stress that have AtHB6-binding sites in their promoters, we selected AtABF4 (ABRE Binding Factor 4) and AtDREB2A (Dehydration-Responsive Element Binding Protein 2) as possible downstream targets of AtHB6 (Chow et al. 2019). In the B. napus genome, BnaABF4 and BnaDREB2A are represented by multiple copies, i.e. six and four duplicates, respectively. We compared the promoter sequences of AtABF4 and AtDREB2A with BnaABF4 and BnaDREB2A homologues, respectively, to identify regions with conserved TF-binding sites (Fig. 6). We found the conserved position of the HB6-binding site in the promoters of four BnaABF4 and two BnaDREB2 homologues. To verify whether the BnaA09HB6 and BnaC08HB6 TFs directly bind to the fragment containing the CAATAATTG cis-element in these promoters of BnaABF4 and BnaDREB2A in vivo, we performed ChIP-qPCR analysis. Our results showed significant enrichment of BnaA09HB6 and BnaC08HB6 in the promoters of four of the six BnaABF4 genes and two of the four BnaDREB2A genes (Fig. 7A and 7B). We found that BnaA09HB6 and BnaC08HB6 enriched the two BnaDREB2A promoters by 3.9- to 5.4-fold and 2.8- to 6.7-fold, respectively. Similarly, BnaA09HB6 and BnaC08HB6 enriched the four BnaABF4 promoters by 3.7- to 4.5-fold and 2.9- to 4.3-fold, respectively. In contrast, we found no significant enrichment of these two BnaHB6 by other BnaABF4 and BnaDREB2A. These results support the conclusion that the BnaA10DREB2A, BnaC09DREB2A, BnaA09ABF4, BnaA03ABF4, BnaA05ABF4 and BnaC05ABF4 genes are direct downstream targets of BnaA09HB6 and BnaC08HB6 in vivo.
Schematic diagram of A. AtABF4 and six BnaABF4 promoters; B. AtDREB2A and four BnaDREB2A promoters, with TFs binding to motifs verified by ChIP-seq experiments and collected in the PlantPAN 3.0 database (Chow et al. 2019; http://plantpan.itps.ncku.edu.tw/). The positions of the predicted binding sites of TFs involved in resistance to osmotic stress by modulating ABA and salt responsiveness are indicated. The legend of the symbols representing potential transcription factors that bind to the sites is given on the right
Characterization of BnaABF4-BnaHB6 and BnaDREB2A-BnaHB6 interactions by ChIP-qPCR analysis. A. Upper panel: schematic diagram of the promoters of BnaA10DREB2A and BnaC09DREB2A with the position of the binding motif recognized by BnaA09HB6 and BnaC08HB6. Bottom: Enrichment of the promoter fragments of BnaA10DREB2A and BnaC09DREB2A with a motif recognized by two BnaA09HB6 and BnaC08HB6 in B. napus. B. Top: Schematic diagram of the promoters of BnaA05ABF4, BnaA03ABF4, BnaC05ABF4 and BnaC03ABF4 indicating the position of the binding element recognized by BnaA09HB6 and BnaC08HB6. Bottom: Enrichment of promoter fragments of BnaA05ABF4, BnaA03ABF4, BnaC05ABF4 and BnaC03ABF4 with a motif recognized by two BnaHB6 in B. napus. Sonicated chromatin was immunoprecipitated with anti-His antibodies. Fragmented genomic DNA was eluted from the protein-DNA complexes and subjected to qPCR analysis. The degree of enrichment of precipitated DNA fragments restricted to regions containing the motif under the study was quantified by qRT-PCR with specific primers. Fold enrichment for each promoter was normalized to the input controls. Vectors were performed as negative controls. The error bars show the standard deviation (SD) for three replicates (n = 3)
The discovered interactions of some BnaABF4 and BnaDREB2A homologues with BnaA09HB6 and BnaC08HB6 suggest that these genes may be involved in the same biological processes. To confirm their functional relationship, we examined their expression profiles under dehydration and salt stress in the wild type (WT) and the bnac08hb6 TILLING mutant (Fig. 8). This line was selected on the basis of possible point mutations in the BnaC08HB6 gene. Based on the SIFT program (http://sift.bii.a-star.edu.sg/; Ng and Henikoff 2003) (Ng and Henikoff 2003), we found five missense mutations (both non-conservative and conservative) and one nonsense mutation that could affect protein function (Fig. S2). After genotyping, the homozygous status of the bnaco8hb6 mutant was confirmed with an abnormal phenotype (Fig. S3). We also observed that loss of BnaC08HB6 function increased the sensitivity of the plant to dehydration and the RWC (Relative Water Content) was lower in the bnaco8hb6 mutant line compared to the WT (Fig. S4). The transcript level of BnaC08HB6 was significantly reduced in the bnac08hb6 mutant compared to the WT plants under both control and stress conditions (Fig. 8). The expression of BnaA09HB6, the orthologous gene of BnaC08HB6, is slightly reduced in the bnac08hb6 mutant under unstressed conditions and significantly reduced under stress. In contrast, the expression of BnaA10DREB2A, BnaC09DREB2A, BnaA05ABF4, BnaA03ABF4 and BnaC03ABF4 increases in the bnac08hb6 mutant compared to WT plants under control conditions. On the other hand, their expression is strongly reduced in the bnac08hb6 mutant under salt stress and dehydration.
Expression patterns of BnaA09HB6, BnaC08HB6, BnaA10DREB2A, BnaC09DREB2A, BnaA05ABF4, BnaC05ABF4, BnaA03ABF4 and BnaC03ABF4 in WT and the bnac08hb6 mutant under dehydration and salt stress. The error bars show the standard deviation (SD) for three replicates (n = 3). Asterisks indicate statistically significant differences calculated with the Student’s t-test (*P < 0.005; **P < 0.001)
Discussion
TFs are important regulators of gene expression that control complex biological processes. The HD-Zip I genes, which belong to the plant-specific HD-Zip gene family, are of great interest as they are involved in the regulation of plant growth and development and respond to stress (Henriksson et al. 2005). Among the 17 members of this subfamily in A. thaliana is the well-characterized AtHB6 gene, which is a negative regulator of the ABA signaling pathway (Söderman et al. 1999; Himmelbach et al. 2002; Fig. 1A). In the present study, we identified and functionally characterized four BnaHB6 homologues from B. napus that represent a lower number of duplicates than those resulting from genome triplication (Chalhoub et al. 2014). This is consistent with previous reports suggesting that some duplicates are lost in the long term due to fractionation followed by diploidization (Lynch and Conery 2000; Kumar et al. 2019; He et al. 2021). The phylogenetic analysis confirmed a close evolutionary relationship between four BnaHB6 homologous proteins and AtHB6, which are grouped in the same clade β2 (Fig. 1B). Among them, BnaA09HB6 and BnaC08HB6 belong to the same cluster as AtHB6, confirming their common origin and suggesting similar functions. BnaA04HB6 and BnaC04HB6, on the other hand, are distantly related to AtHB6 and probably originate from an additional whole genome triplication that occurred in the Brassica-specific lineage (The Brassica rapa Genome Sequencing Project Consortium et al. 2011). All BnaHB6 show transcriptional activity in yeast, so they may act as TFs to regulate downstream gene expression (Fig. 2). However, the differences in transcriptional activity between the orthologous gene pairs BnaA04HB6/BnaC04HB6 and BnaA09HB6/BnaC08HB6 were observed in the quantitative β-galactosidase assay. Previous studies have shown that changes in orthologous TFs contribute to the evolution of the regulatory network, affecting DNA-binding specificity and leading to phenotypic diversity (Price et al. 2007; Gabaldón and Koonin 2013; Nadimpalli et al. 2015). Therefore, further research should focus on investigating the regulatory interactions and expression patterns of these genes to understand their effects on phenotype. To determine the functional redundancy or diversity of the four BnaHB6 homologues, we compared their sequences and motif organization with each other and with AtHB6 and then studied their expression patterns in different tissues, at early developmental stages, under stress conditions and in circadian rhythm (Figs. 3 and S1). We observed differences in the sequences of introns and exons corresponding to the C- and N-terminal regions of the BnaHB6 proteins that affect the arrangement of regulatory motifs (Fig. S1). These changes may be responsible for their functional diversity, with the BnaHB6 homologues interacting with different target partners and regulating different signaling pathways. Previous reports have highlighted the likely role of additional regulatory motifs in the C- and N-terminal regions of HD-Zip I proteins in their functional divergence (Arce et al. 2011; Capella et al. 2014). Expression analyzes of the BnaHB6 genes confirmed their functional complexity in growth and development processes as well as in the stress response (Fig. 3). They show similar expression patterns at early developmental stages and in the tissues studied, suggesting that they may have redundant functions in regulating these processes (Fig. 3A). On the other hand, only two of them, namely BnaA09HB6 and BnaC08HB6, are involved in the response to dehydration and salt stress via the ABA signaling pathway and they are induced in the dark (Figs. 1B, 3B and 3C). Previous studies have found a link between the circadian clock and changes in the expression of genes involved in the response to abiotic stresses such as heat, cold and drought (Mizuno and Yamashino 2008; Wilkins et al. 2010). Our data confirm that the time of day of induction may be one of the factors responsible for the differential stress response of BnaHB6 genes. The functions of some HD-Zip I genes in light-induced plant growth and development has been also postulated (Aoyama et al. 1995; Wang et al. 2003; Henriksson et al. 2005; Manavella et al. 2008; Choi et al. 2014). To date, the role of the HB6 gene in the circadian rhythm and in the regulation of light-dependent developmental processes remains unknown.
Phylogenetic analyzes allowed us not only to determine the evolutionary relationship between the members of the gene subfamily, but also to speculate on their putative functions. Previous reports have shown that evolutionarily related HD-Zip I genes in different species (evolved from speciation) have similar functions in plant growth and development as well as in response to abiotic stress (Ariel et al. 2010; Harris et al. 2011; Wei et al. 2019; González et al. 2019). In Arabidopsis, AtHB6 is located together with AtHB16 and AtHB5 in the clade β2 and they have evolved through gene duplications (Henriksson et al. 2005; Fig. 1A). In the B. napus genome, the BnaHB6, BnaHB16 and BnaHB5 genes each have four homologues are clustered in the clade β2, suggesting their functional redundancy in developmental processes and stress responses (Fig. 1B). Our data show the functional complexity of the BnaHB6, BnaHB16 and BnaHB5 genes at early developmental stages and under stress conditions with partially overlapping functions (Fig. 3). These results suggest that duplications play a role in the functional diversity of BnaHB genes belonging to the clade β2. This is consistent with previous studies showing functional divergence in members of other gene families such as PP2CA, CDPK and COL in the Brassica species (Ludwików et al. 2013; Zhang et al. 2014; Babula-Skowrońska 2021).
To better understand the mechanisms that regulate the expression of the BnaHB6 homologues at the transcriptional level, we compared the number and localization of cis-regulatory elements in the AtHB6 and the four BnaHB6 promoters that might affect the expression activity and functional specificity of these genes in the stress response (Fig. 4). The observed differences in the number and localization of stress-responsible cis-acting elements in their promoters, with the promoters of AtHB6, BnaA09HB6 and BnaC08HB6 being more conserved, confirm that both BnaHB6 genes are involved in the response to dehydration and salt stress. Using transgenic A. thaliana plants expressing the GUS reporter gene under the control of the individual BnaHB6 promoter, we observed GUS activity of the orthologous genes BnaA09HB6 and BnaC08HB6 in the columella root cap and in the root core including the xylem (Fig. 5B). Previous molecular studies have shown that columella cells contain amyloplasts and can increase the root angle in the direction of gravity in root tips (Ge and Chen 2019). They are also involved in deepening the root structure during drought. The root xylem, on the other hand, is involved in the transport of water from the soil and from cell to cell, among other things.
In this study, we also gained insight into the role of BnaHB6 genes of B. napus in the regulation of downstream target genes under dehydration and salt stress. Based on experimentally verified data on TF-binding sites in genes involved in drought, salt stress and ABA in A. thaliana, we selected AtABF4 and AtDREB2A, whose promoters have AtHB6-binding sites (Chow et al. 2019). Consistent with the duplicated structure of the B. napus genome, the BnaABF4 and BnaDREB2A genes are represented by a larger number of homologues, only some of which have BnaHB6-binding sites in their promoters (Fig. 6A and 6B).
We found that BnaA09HB6 and BnaC08HB6 bind directly to only four of six BnaABF4 homologues and two of four BnaDREB2A to regulate their transcription (Fig. 7A and 7B). These results indicate that WGD, followed by genomic rearrangements, has led to changes in DNA-binding preferences. The discovered interactions between some homologues of BnaABF4 and BnaDREB2A as well as BnaA09HB6 and BnaC08HB6 suggest that these genes may be involved in regulating the same biological processes. To determine the functional relationship between these genes, we examined their expression under dehydration and salt stress in the WT and the bnac08hb6 TILLING mutant (Fig. 8). The bnaco8hb6 mutant showed an abnormal phenotype with shortened root length and fleshy, thickened leaves (Fig. S3). In addition, loss of BnaC08HB6 function increased the plant’s sensitivity to dehydration and decreased RWC compared to the WT (Fig. S4). Hence, BnaC08HB6 is positively involved in the developmental processes independent of environmental changes and response to abiotic stress. We found that BnaABF4 and BnaDREB2A as well as BnaA09HB6 and BnaC08HB6 were induced in the WT plants under both control and stress conditions and in the bnac08hb6 mutant only under control conditions, with the exception of BnaC05ABF4. These results confirm that BnaC08A09HB6 can positively regulate the expression of some BnaABF4 and BnaDREB2A targets under stress conditions.
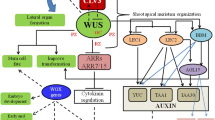
In conclusion, our results in this study provide new insights into the functional diversity of four BnaHB6 homologues in regulating the stress response. First, we show the different transcriptional responses of the BnaHB6 homologues under dehydration and salt stress as well as under light conditions (Fig. 9). We have identified BnaC08HB6 and BnaA09HB6 as molecular components acting downstream of the selected BnaABF2 and BnaDREB2A in regulating the response to dehydration and salt stress. This is a first step towards understanding the differences in the gene regulatory network activated by individual BnaHB6 homologues in response to stress.
Working model of the functional diversity of the BnaHB6 homologues in stress response and circadian rhythm and in the regulation of BnaABF and BnaDREB2A genes under dehydration and salt stress; Left: two of four BnaHB6 homologues (BnaA09HB6 and BnaC09HB6) are induced in an ABA manner under dehydration and salt stress and they directly upregulate the expression of some BnaABF and BnaDREB2A homologues under these stress conditions by binding to their promoters; Right: changes in the expression of four BnaHB6 genes in a 24-h cycle with 16 h of light and 8 h of darkness
Data availability
All data generated or analyzed during this study re included in this published article or as supplementary materials.
References
Aoyama T, Dong CH, Wu Y et al (1995) Ectopic expression of the Arabidopsis transcriptional activator Athb-1 alters leaf cell fate in tobacco. Plant Cell 7:1773–1785. https://doi.org/10.1105/tpc.7.11.1773
Arce AL, Raineri J, Capella M et al (2011) Uncharacterized conserved motifs outside the HD-Zip domain in HD-Zip subfamily I transcription factors; a potential source of functional diversity. BMC Plant Biol 11:42. https://doi.org/10.1186/1471-2229-11-42
Ariel FD, Manavella PA, Dezar CA, Chan RL (2007) The true story of the HD-Zip family. Trends Plant Sci 12:419–426. https://doi.org/10.1016/j.tplants.2007.08.003
Ariel F, Diet A, Verdenaud M, Crespi M (2010) Environmental regulation of lateral root emergence in Medicago truncatula requires the HD-Zip I transcription factor HB1. Plant Cell 22:2171–2183. https://doi.org/10.1105/tpc.110.074823
Babula-Skowrońska D (2021) Functional divergence of Brassica napus BnaABI1 paralogs in the structurally conserved PP2CA gene subfamily of Brassicaceae. Genomics 113:3185–3197. https://doi.org/10.1016/j.ygeno.2021.06.031
Babula-Skowrońska D, Ludwików A, Cieśla A et al (2015) Involvement of genes encoding ABI1 protein phosphatases in the response of Brassica napus L. to drought stress. Plant Mol Biol 88:445–457. https://doi.org/10.1007/s11103-015-0334-x
Capella M, Ré DA, Arce AL, Chan RL (2014) Plant homeodomain-leucine zipper I transcription factors exhibit different functional AHA motifs that selectively interact with TBP or/and TFIIB. Plant Cell Rep 33:955–967. https://doi.org/10.1007/s00299-014-1576-9
Capella M, Ribone PA, Arce AL, Chan RL (2015) Arabidopsis thaliana HomeoBox 1 (AtHB1), a Homedomain-Leucine Zipper I (HD-Zip I) transcription factor, is regulated by PHYTOCHROME-INTERACTING FACTOR 1 to promote hypocotyl elongation. New Phytol 207:669–682. https://doi.org/10.1111/nph.13401
Chalhoub B, Denoeud F, Liu S et al (2014) Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 345:950–953. https://doi.org/10.1126/science.1253435
Chen X, Truksa M, Shah S, Weslake RJ (2010) A survey of quantitative real-time polymerase chain reaction internal reference genes for expression studies in Brassica napus. Anal Biochem 405:138–140. https://doi.org/10.1016/j.ab.2010.05.032
Choi H, Jeong S, Kim DS et al (2014) The homeodomain-leucine zipper ATHB23, a phytochrome B-interacting protein, is important for phytochrome B-mediated red light signaling. Physiol Plant 150:308–320. https://doi.org/10.1111/ppl.12087
Chow C-N, Lee T-Y, Hung Y-C et al (2019) PlantPAN3.0: a new and updated resource for reconstructing transcriptional regulatory networks from ChIP-seq experiments in plants. Nucleic Acids Res 47:D1155–D1163. https://doi.org/10.1093/nar/gky1081
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743. https://doi.org/10.1046/j.1365-313x.1998.00343.x
Gabaldón T, Koonin E (2013) Functional and evolutionary implications of gene orthology. Nat Rev Genet 14:360–366. https://doi.org/10.1038/nrg3456
Ge L, Chen R (2019) Negative gravitropic response of roots directs auxin flow to control root gravitropism. Plant Cell Environ 42:2372–2383. https://doi.org/10.1111/pce.13559
González FG, Capella M, Ribichich KF et al (2019) Field-grown transgenic wheat expressing the sunflower gene HaHB4 significantly outyields the wild type. J Exp Bot 70:1669–1681. https://doi.org/10.1093/jxb/erz037
Harris JC, Harris JC, Hrmova M et al (2011) Tansley review modulation of plant growth by HD-Zip class I and II transcription factors in response to environmental stimuli. New Phytol. https://doi.org/10.1111/j.1469-8137.2011.03733.x
He Z, Ji R, Havlickova L et al (2021) Genome structural evolution in Brassica crops. Nat Plants 7:757–765. https://doi.org/10.1038/s41477-021-00928-8
Henriksson E, Olsson ASB, Johannesson H et al (2005) Homeodomain leucine zipper class I genes in Arabidopsis. Expression patterns and phylogenetic relationships. Plant Physiol 139:509–518. https://doi.org/10.1104/pp.105.063461
Himmelbach A, Hoffmann T, Leube M, Höhener B, Grill E (2002) Homeodomain protein ATHB6 is a target of the protein phosphatase ABI1 and regulates hormone responses in Arabidopsis. EMBO J 21:3029–3038. https://doi.org/10.1093/emboj/cdf316
Jiang J, Wang Y, Zhu B et al (2015) Digital gene expression analysis of gene expression differences within Brassica diploids and allopolyploids. BMC Plant Biol 15:1–13. https://doi.org/10.1186/s12870-015-0417-5
Jing Z, Duan W, Song X et al (2017) Preferential retention, expression profile and potential functional diversity analysis of HD-Zip gene family in Brassica rapa. J Plant Growth Regul 82:421–430. https://doi.org/10.1007/s10725-017-0268-1
Johannesson H, Wang Y, Engström P (2001) DNA-binding and dimerization preferences of Arabidopsis homeodomain-leucine zipper transcription factors in vitro. Plant Mol Biol 45:63–73. https://doi.org/10.1023/A:1006423324025
Khan N, Hu C, Khan WA et al (2018) Genome-wide identification, classification, and expression pattern of homeobox gene family in Brassica rapa under various stresses. Sci Rep 8:16265. https://doi.org/10.1038/s41598-018-34448-x
Kumar R, Lee SG, Augustine R et al (2019) Molecular basis of the evolution of methylthioalkylmalate synthase and the diversity of methionine-derived glucosinolates. Plant Cell 31:1633–1647. https://doi.org/10.1105/tpc.19.00046
Lin Z, Hong Y, Yin M et al (2008) A tomato HD-Zip homeobox protein, LeHB-1, plays an important role in floral organogenesis and ripening. Plant J 55:301–310. https://doi.org/10.1111/j.1365-313X.2008.03505.x
Liu W, Fu R, Li Q et al (2013) Genome-wide identification and expression profile of homeodomain-leucine zipper Class I gene family in Cucumis sativus. Gene 531:279–287. https://doi.org/10.1016/j.gene.2013.08.089
Ludwików A, Babula-Skowrońska D, Szczepaniak M et al (2013) Expression profiles and genomic organisation of group A protein phosphatase 2C genes in Brassica oleracea. Ann Appl Biol 163:124–134. https://doi.org/10.1111/aab.12039
Lynch M, Conery JS (2000) The evolutionary fate and consequences of duplicate genes. Science 290:1151–1155. https://doi.org/10.1126/science.290.5494.1151
Manavella PA, Dezar CA, Ariel FD et al (2008) The sunflower HD-Zip transcription factor HAHB4 is up-regulated in darkness, reducing the transcription of photosynthesis-related genes. J Exp Bot 59:3143–3155. https://doi.org/10.1093/jxb/ern170
Mizuno T, Yamashino T (2008) Comparative transcriptome of diurnally oscillating genes and hormone-responsive genes in Arabidopsis thaliana: insight into circadian clock-controlled daily responses to common ambient stresses in plants. Plant Cell Physiol 49:481–487. https://doi.org/10.1093/pcp/pcn008
Mooney BC, Graciet E (2020) A simple and efficient Agrobacterium-mediated transient expression system to dissect molecular processes in Brassica rapa and Brassica napus. Plant Direct 4:e00237. https://doi.org/10.1002/pld3.237
Nadimpalli S, Persikov AV, Singh M (2015) Pervasive variation of transcription factor orthologs contributes to regulatory network evolution. PLoS Genet 11:e1005011. https://doi.org/10.1371/journal.pgen.1005011
Ng PC, Henikoff S (2003) SIFT: Predicting amino acid changes that affect protein function. Nucl Acids Res 31:3812–3814. https://doi.org/10.1093/nar/gkg509
Olsson ASB, Engström P, Söderman E (2004) The homeobox genes ATHB12 and ATHB7 encode potential regulators of growth in response to water deficit in Arabidopsis. Plant Mol Biol 55:663–677. https://doi.org/10.1007/s11103-004-1581-4
Ostergaard L, King GJ (2008) Standardized gene nomenclature for the Brassica genus. Plant Methods 4:10. https://doi.org/10.1186/1746-4811-4-10
Palena CM, Gonzalez DH, Chan RL (1999) A monomer-dimer equilibrium modulates the interaction of the sunflower homeodomain leucine-zipper protein Hahb-4 with DNA. Biochem J 341:81–87. https://doi.org/10.1042/bj3410081
Parkin IAP, Gulden SM, Sharpe AG et al (2005) Segmental structure of the Brassica napus genome based on comparative analysis with Arabidopsis thaliana. Genetics 171:765–781. https://doi.org/10.1534/genetics.105.042093
Peng X, Wu D, Zhang X et al (2022) Identification and characterization of the HD-Zip gene family and dimerization analysis of HB7 and HB12 in Brassica napus L. Genes 13:2139. https://doi.org/10.3390/genes13112139
Perotti MF, Ribone PA, Cabello JV et al (2019) AtHB23 participates in the gene regulatory network controlling root branching, and reveals differences between secondary and tertiary roots. Plant J 100:1224–1236. https://doi.org/10.1111/tpj.14511
Perotti MF, Arce AL, Chan RL (2021) The underground life of homeodomain-leucine zipper transcription factors. J Exp Bot 72:4005–4021. https://doi.org/10.1093/jxb/erab112
Price MN, Dehal PS, Arkin AP (2007) Orthologous transcription factors in bacteria have different functions and regulate different genes. PLoS Comput Biol 3:e175. https://doi.org/10.1371/journal.pcbi.0030175
Ré DA, Capella M, Bonaventure G, Chan RL (2014) Arabidopsis AtHB7 and AtHB12 evolved divergently to fine tune processes associated with growth and responses to water stress. BMC Plant Biol 14:1–14. https://doi.org/10.1186/1471-2229-14-150
Ribone PA, Capella M, Chan RL (2015) Functional characterization of the homeodomain leucine zipper i transcription factor AtHB13 reveals a crucial role in Arabidopsis development. J Exp Bot 66:5929–5943. https://doi.org/10.1093/jxb/erv302
Sessa G, Morelli G, Ruberti I (1993) The Athb-1 and -2 HD-Zip domains homodimerize forming complexes of different DNA binding specificities. EMBO J 12:3507–3517. https://doi.org/10.1002/j.1460-2075.1993.tb06025.x
Söderman E, Hjellström M, Fahleson J, Engström P (1999) The HD-Zip gene ATHB6 in Arabidopsis is expressed in developing leaves, roots and carpels and up-regulated by water deficit conditions. Plant Mol Biol 40:1073–1083. https://doi.org/10.1023/A:1006267013170
The Brassica rapa Genome Sequencing Project Consortium et al (2011) The genome of the mesopolyploid crop species Brassica rapa. Nat Genet 43:1035–1039. https://doi.org/10.1038/ng.919
Wang Y, Henriksson E, Söderman E et al (2003) The Arabidopsis homeobox gene, ATHB16, regulates leaf development and the sensitivity to photoperiod in Arabidopsis. Develop Biol 264:228–239. https://doi.org/10.1016/j.ydbio.2003.07.017
Wei M, Liu A, Zhang Y et al (2019) Genome-wide characterization and expression analysis of the HD-Zip gene family in response to drought and salinity stresses in sesame. BMC Genomics 20:1–13. https://doi.org/10.1186/s12864-019-6091-5
Wilkins O, Bräutigam K, Campbell MM (2010) Time of day shapes Arabidopsis drought transcriptomes. Plant J 63:715–727. https://doi.org/10.1111/j.1365-313X.2010.04274.x
Yang Y, Al-baidhani HHJ, Harris J et al (2020) DREB/CBF expression in wheat and barley using the stress-inducible promoters of HD-Zip I genes: impact on plant development, stress tolerance and yield. Plant Biotechnol J. 2:829–844. https://doi.org/10.1111/pbi.13252
Yin L, Sun Y, Chen X et al (2023) Genome-wide analysis of the HD-Zip gene family in Chinese Cabbage (Brassica rapa subsp. pekinensis) and the expression pattern at high temperatures and in carotenoids regulation. Agronomy 13:1324. https://doi.org/10.3390/agronomy13051324
Zhang Z, Chen X, Guan X et al (2014) A genome-wide survey of homeodomain-leucine zipper genes and analysis of cold-responsive HD-Zip I members expression in tomato. Biosci Biotech Biochem 78:1337–1349. https://doi.org/10.1080/09168451.2014.923292
Zhang J, Zhu Q-H, Moncuquet P et al (2016) Genome-wide identification and characterization of the homeodomain-leucine zipper I family of genes in cotton (Gossypium spp.). Plant Gene 7:50–61. https://doi.org/10.1016/j.plgene.2016.05.002
Żyła N, Babula-Skowrońska D (2023) Evolutionary consequences of functional and regulatory divergence of HD-Zip I transcription factors as a source of diversity in protein interaction networks in plants. J Mol Evol 91:581–597. https://doi.org/10.1007/s00239-023-10121-4
Acknowledgements
We thank prof. Ian Bancroft of University of York, UK for providing the transgenic lines from the B. napus TILLING population and John Innes Centre, Norwich, UK for providing pBRACT vectors. This work was funded by grant from the National Science Centre 2016/23/B/N29/02175 to DBS.
Funding
Narodowe Centrum Nauki, 2016/23/B/N29/02175, Danuta Babula-Skowronska
Author information
Authors and Affiliations
Contributions
DBS conceived the project, designed the research, conducted the experiments, analyzed the data and wrote the manuscript. NŻ performed the experiments, analyzed the data and wrote the manuscript. AC and LS conducted the experiments. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Żyła, N., Cieśla, A., Szała, L. et al. Functional and regulatory diversity of homeobox-leucine zipper transcription factors BnaHB6 under dehydration and salt stress in Brassica napus L.. Plant Mol Biol 114, 59 (2024). https://doi.org/10.1007/s11103-024-01465-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11103-024-01465-6