Abstract
Key message
Sorghum glycine rich proline rich protein (SbGPRP1) exhibit antimicrobial properties and play a crucial role during biotic stress condition.
Abstract
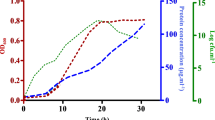
Several proteins in plants build up the innate immune response system in plants which get triggered during the occurrence of biotic stress. Here we report the functional characterization of a glycine-rich proline-rich protein (SbGPRP1) from Sorghum which was previously demonstrated to be involved in abiotic stresses. Expression studies carried out with SbGPRP1 showed induced expression upon application of phytohormones like salicylic acid which might be the key in fine-tuning the expression level. Upon challenging the Sorghum plants with a compatible pathogen the SbGprp1 transcript was found to be upregulated. SbGPRP1 encodes a 197 amino acid polypeptide which was bacterially-expressed and purified for in vitro assays. Gram-positive bacteria like Bacillus and phytopathogen Rhodococcus fascians showed inhibited growth in the presence of the protein. The NPN assay, electrolytic leakage and SEM analysis showed membrane damage in bacterial cells. Ectopic expression of SbGPRP1 in tobacco plants led to enhanced tolerance towards infection caused by R. fascians. Though the N-terminal part of the protein showed disorderness the C-terminal end was quite capable of forming several α-helices which was correlated with CD spectroscopic analysis. Here, we have tried to determine the structural model for the protein and predicted the association of antimicrobial activity with the C-terminal region of the protein.







Similar content being viewed by others
References
Agarwal T, Upadhyaya G, Halder T, Mukherjee A, Majumder AL, Ray S (2017) Different dehydrins perform separate functions in Physcomitrella patens. Planta 245(1):101–118
Altschul Stephen F, Warren G, Webb M, Eugene WM, David JL (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410
Berman HM (2008) The protein data bank: a historical perspective. Acta Crystallogr A 64:88–95
Blank M, Yehuda S (2008) Histidine-rich glycoprotein modulation of immune/autoimmune, vascular, and coagulation systems. Clin Rev Allergy Immunol 34(3):307–312
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brooks BR, Charles L, Brooks III, Alexander D, Jr Mackerell et al (2009) CHARMM: the biomolecular simulation program. J Comput Chem 30(10):1545–1614
Cheung AY, May B, Kawata EE, Gu Q, Wu H-M (1993) Characterization of cDNAs for stylar transmitting tissue-specific proline-rich proteins in tobacco. Plant J 3(1):151–160
Corpet F (1988) Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 16:10881–10890
Croes S, Weyens N, Colpaer J, Vangronsveld J (2015) Characterization of the cultivable bacterial populations associated with field grown Brassica napus L.: an evaluation of sampling and isolation protocols. Environ Microbiol 17(7):2379–2392
de O Manes CL, van Montagu M, Prinsen E, Goethals K, Holsters M (2001) De novo cortical cell division triggered by the phytopathogen Rhodococcus fascians in tobacco. Mol Plant Microbe Interact 14(2):189–195
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 1(4):19–21
Dinneny JR, Long TA, Wang JY, Jung JW, Mace D, Pointer S et al (2008) Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science 320:942–945
Edmunds LK, Zummo N (1975) Sorghum diseases in the United States and their control. Sorghum diseases in the United States and their control. (468)
Eisenberg D, Roland L, James UB (1997) VERIFY3D: assessment of protein models with three-dimensional profiles. Methods Enzymol 277:396–404
Epand RM, Vogel HJ (1999) Diversity of antimicrobial peptides and their mechanisms of action. Biochim Biophys Acta 1462(1):11–28
Feng Y, Peng H, Liang S (2011) Molecular analysis of the PGYRP (proline-, glycine- and tyrosine-rich protein) gene family in soybean. Mol Biol Rep 38:2739–2750
Fischer ER, Hansen BT, Nair V, Hoyt FH, Dorward DW (2012) Scanning electron microscopy. Curr Protoc Microbiol 1:2. https://doi.org/10.1002/9780471729259.mc02b02s25
Fiser A, Andrej S (2003) ModLoop: automated modeling of loops in protein structures. Bioinformatics 19(18):2500–2501
Fiser A, Do RK, Sali A (2000) Modeling of loops in protein structures. Protein Sci 9(9):1753–1773
Franssen HJ, Nap JP, Gloudemans T, Stiekema W, Van Dam H, Govers F et al (1987) Characterization of cDNA for nodulin-75 of soybean: a gene product involved in early stages of root nodule development. Proc Natl Acad Sci 84(13):4495–4499
Geli MI, Torrent M, Ludevid D (1994) Two structural domains mediate two sequential events in [gamma]-zein targeting: protein endoplasmic reticulum retention and protein body formation. Plant Cell 6(12):1911–1922
Halder T, Agarwal T, Ray S (2016) Isolation, cloning, and characterization of a novel Sorghum dehydrin (SbDhn2) protein. Protoplasma 253(6):1475–1488
Halder T, Upadhyaya G, Ray S (2017) YSK2 type Dehydrin (SbDhn1) from Sorghum bicolor showed improved protection under high temperature and osmotic stress condition. Front Plant Sci 8:918
Hampton CR, Bowen HC, Broadley MR, Hammond JP, Mead A, Payne KA et al (2004) Cesium toxicity in Arabidopsis. Plant Physiol 136:3824–3837
Herbel V, Schäfer H, Wink M (2015) Recombinant production of Snakin-2 (an antimicrobial peptide from tomato) in E. coli and analysis of its bioactivity. Molecules 20(8):14889–14901
Herbel V, Sieber-Frank J, Wink M (2017) The antimicrobial peptide snakin-2 is upregulated in the defense response of tomatoes (Solanum lycopersicum) as part of the jasmonate-dependent signaling pathway. J Plant Physiol 208:1–6
Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231
Jenssen H, Hamill P, Hancock RE (2006) Peptide antimicrobial agents. Clin Microbiol Rev 19(3):491–511
Jose-Estanyol M, Ruiz-Avila L, Puigdomènech P (1992) A maize embryo-specific gene encodes a proline-rich and hydrophobic protein. Plant Cell 4(4):413–423
Katile SO, Perumal R, Rooney WL, Prom LK, Magill CW (2010) Expression of pathogenesis-related protein PR-10 in sorghum floral tissues in response to inoculation with Fusarium thapsinum and Curvularia lunata. Mol Plant Pathol 11(1):93–103
Kay BK, Williamson MP, Sudol M (2000) The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J 14(2):231–241
Lee BH, Henderson DA, Zhu JK (2005) The Arabidopsis cold-responsive transcriptome and its regulation by ICE1. Plant Cell 17:3155–3175
Lovell SC, Davis IW, Arendall WB III, De Bakker PI, Word JM, Prisant MG et al (2003) Structure validation by Cα geometry: ϕ, ψ and Cβ deviation. Proteins 50(3):437–450
Lüthy R, Bowie JU, Eisenberg D (1992) Assessment of protein models with three-dimensional profiles. Nature 356(6364):83
Marty I, Monfort A, Stiefel V, Ludevid D, Delseny M, Puigdomenech P (1996) Molecular characterization of the gene coding for GPRP, a class of proteins rich in glycine and proline interacting with membranes in Arabidopsis thaliana. Plant Mol Biol 30:625–636
Matsushima N, Creutz CE, Kretsinger RH (1990) Polyproline, beta-turn helices. Novel secondary structures proposed for the tandem repeats within rhodopsin, synaptophysin, synexin, gliadin, RNA polymerase II, hordein, and gluten. Proteins 7:125–155
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Nakahara KS, Kitazawa H, Atsumi G, Choi SH, Suzuki Y, Uyeda I (2011) Screening and analysis of genes expressed upon infection of broad bean with Clover yellow vein virus causing lethal necrosis. Virol J 8:355
Pathan AK, Bond J, Gaskin RE (2010) Sample preparation for SEM of plant surfaces. Mater Today 12:32–43
Peng H, Feng Y, Zhang H, Wei X, Liang S (2012) Molecular cloning and characterisation of genes coding for glycine- and proline-rich proteins (GPRPs) in soybean. Plant Mol Biol Rep 30:566–577
Pertry I, Václavíková K, Gemrotová M, Spíchal L, Galuszka P, Depuydt S et al (2010) Rhodococcus fascians impacts plant development through the dynamic fas-mediated production of a cytokinin mix. Mol Plant Microbe Interact 23(9):1164–1174
Pommerrenig B, Barth I, Niedermeier M, Kopp S, Schmid J, Dwyer RA et al (2006) Common plantain. A collection of expressed sequence tags from vascular tissue and a simple and efficient transformation method. Plant Physiol 142(4):1427–1441
Rao X, Xuelin H, Zhicheng Z, Xin L (2013) An improvement of the 2ˆ (–delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinform Biomath 3(3):71
Raussens V, Ruysschaert JM, Goormaghtigh E (2003) Protein concentration is not an absolute prerequisite for the determination of secondary structure from circular dichroism spectra: a new scaling method. Anal Biochem 319(1):114–121
Rollema HS, Kuipers OP, Both P, De Vos WM, Siezen RJ (1995) Improvement of solubility and stability of the antimicrobial peptide nisin by protein engineering. Appl Environ Microbiol 61(8):2873–2878
Sachetto-Martins G, Franco LO, de Oliveira DE (2000) Plant glycine-rich proteins: a family or just proteins with a common motif? Biochim Biophys Acta 1492(1):1–14
Shen MY, Sali A (2006) Statistical potential for assessment and prediction of protein structures. Protein Sci 15(11):2507–2524
Smith RH, Bhaskaran S (1986) Sorghum [Sorghum bicolor (L.) Moench]. In: Bajaj YPS (ed) Crops I. biotechnology in agriculture and forestry, vol 2. Springer, Berlin
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0 Mol. Biol Evol 30:2725–2729
Thomas S, Karnik S, Barai RS, Jayaraman VK, Idicula-Thomas S (2009) CAMP: a useful resource for research on antimicrobial peptides. Nucleic Acids Res 38:D774–D780
Tossi A, Sandri L, Giangaspero A (2000) Amphipathic, α-helical antimicrobial peptides. Pept Sci 55(1):4–30
Vandeputte O, Öden S, Mol A, Vereecke D, Goethals K, Jaziri El et al (2005) Biosynthesis of auxin by the gram-positive phytopathogen Rhodococcus fascians is controlled by compounds specific to infected plant tissues. Appl Environ Microbiol 71(3):1169–1177
Vereecke D, Burssens S, Simón-Mateo C, Inzé D, Van Montagu M, Goethals K, Jaziri M (2000) The Rhodococcus fascians-plant interaction: morphological traits and biotechnological applications. Planta 210(2):241–251
Waghu FH, Gopi L, Barai RS, Ramteke P, Nizami B, Idicula-Thomas S (2014) CAMP: collection of sequences and structures of antimicrobial peptides. Nucleic Acids Res 42(D1):D1154–D1158
Waghu FH, Barai RS, Gurung P, Idicula-Thomas S (2015) CAMPR3: a database on sequences, structures and signatures of antimicrobial peptides. Nucleic Acids Res 44(D1):D1094–D1097
Wang G, Li X, Wang Z (2015) APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res 44(D1):D1087–D1093
Xie YR, Chen ZY, Brown RL, Bhatnagar D (2010) Expression and functional characterization of two pathogenesis-related protein 10 genes from Zea mays. J Plant Physiol 167(2):121–130
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415(6870):389
Acknowledgements
We express our wholehearted thanks to DST-FIST and UGC CAS for instrumental facilities of Department of Botany, University of Calcutta. We thank CRNN-University of Calcutta for their support regarding scanning electron microscopy. This work is supported by grants to SR from the Council of Scientific and Industrial Research, Government of India (38(1402)/15/EMR-II dated 14.08.2015)) and research fellowship to TH (Sanction No. 09/028(0914)/2013-EMR-I). GU thanks the Department of Science and Technology, Government of India for Research Fellowship (Sanction No. DST/INSPIRE Fellowship/2015/IF150503). AD and ShR thank the University Grants Commission, Government of India for Research Fellowship Sanction No. (813/(CSIR-UGC NET DEC. 2016)), (2061530629 dated 10/12/2015) respectively.
Author information
Authors and Affiliations
Contributions
SR conceived the original screening and research plans and supervised the experiments; TH, GU, ShR, RB, AD and TA. performed most of the experiments; TH, GU, ShR, RB, AB and AD designed the experiments and analyzed the data; SR conceived the project and wrote the article with contributions of all the authors; SR supervised and complemented the writing. TH and GU equally contributed to the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors decalre that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Halder, T., Upadhyaya, G., Roy, S. et al. Glycine rich proline rich protein from Sorghum bicolor serves as an antimicrobial protein implicated in plant defense response. Plant Mol Biol 101, 95–112 (2019). https://doi.org/10.1007/s11103-019-00894-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-019-00894-y