Abstract
Purpose
Pegvisomant (PEG) is an effective therapy for acromegaly. Its safety in women seeking fertility and during pregnancy has been scarcely reported.
Methods
A retrospective chart review was performed in three patients with acromegaly who received PEG while attempting to conceive. Published studies regarding this topic were analyzed.
Results
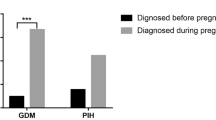
Four pregnancies in three women with acromegaly are reported. In the first patient, PEG was withdrawn three days before embryo transfer in her first pregnancy and 2 weeks prior to transfer in the second pregnancy. Each transfer resulted in a healthy full-term newborn. In the second and third patients, PEG was withdrawn at diagnosis of pregnancy. No fetal complications occurred during gestations which resulted in three full-term newborns (one single and one twin pregnancy). No abnormalities in development were found in the five live births described. Few cases of pregnancies in women exposed to PEG have been reported and therefore safety cannot be clearly established. In this series, all four pregnancies had good outcomes with discontinuation of the drug before or at first knowledge of conception. A review of the literature reveals no evident drug-related abnormalities in the offspring, even in the few women with continued use of PEG throughout pregnancy.
Conclusion
Preconception therapy with PEG resulted in successful fertility outcomes. Although few cases have been reported, these four pregnancies with PEG use prior to or at the time of conception were not associated with significant maternal or fetal complications. More studies are needed to establish the safety of PEG preconception.

Similar content being viewed by others
Data availability
All data is available in the text.all data is available in the text.
Code availability
Not applicable.
References
Abucham J, Bronstein MD, Dias ML (2017) Management of endocrine disease: acromegaly and pregnancy: a contemporary review. Eur J Endocrinol 177(1):R1–r12. https://doi.org/10.1530/eje-16-1059
Kaltsas GA, Mukherjee JJ, Jenkins PJ, Satta MA, Islam N, Monson JP, Besser GM, Grossman AB (1999) Menstrual irregularity in women with acromegaly. J Clin Endocrinol Metab 84(8):2731–2735. https://doi.org/10.1210/jcem.84.8.5858
Katznelson L, Kleinberg D, Vance ML, Stavrou S, Pulaski KJ, Schoenfeld DA, Hayden DL, Wright ME, Woodburn CJ, Klibanski A (2001) Hypogonadism in patients with acromegaly: data from the multi-centre acromegaly registry pilot study. Clin Endocrinol 54(2):183–188. https://doi.org/10.1046/j.1365-2265.2001.01214.x
Grynberg M, Salenave S, Young J, Chanson P (2010) Female gonadal function before and after treatment of acromegaly. J Clin Endocrinol Metab 95(10):4518–4525. https://doi.org/10.1210/jc.2009-2815
Dogansen SC, Tanrikulu S, Yalin GY, Yarman S (2018) Female gonadal functions and ovarian reserve in patients with acromegaly: experience from a single tertiary center. Endocrine 60(1):167–174. https://doi.org/10.1007/s12020-018-1540-5
Petrossians P, Daly AF, Natchev E, Maione L, Blijdorp K, Sahnoun-Fathallah M, Auriemma R, Diallo AM, Hulting AL, Ferone D, Hana V Jr, Filipponi S, Sievers C, Nogueira C, Fajardo-Montanana C, Carvalho D, Hana V, Stalla GK, Jaffrain-Rea ML, Delemer B, Colao A, Brue T, Neggers S, Zacharieva S, Chanson P, Beckers A (2017) Acromegaly at diagnosis in 3173 patients from the Liege Acromegaly Survey (LAS) database. Endocr Relat Cancer 24(10):505–518. https://doi.org/10.1530/erc-17-0253
Clemmons DR, Chihara K, Freda PU, Ho KK, Klibanski A, Melmed S, Shalet SM, Strasburger CJ, Trainer PJ, Thorner MO (2003) Optimizing control of acromegaly: integrating a growth hormone receptor antagonist into the treatment algorithm. J Clin Endocrinol Metab 88(10):4759–4767. https://doi.org/10.1210/jc.2003-030518
Kaltsas GA, Androulakis II, Tziveriotis K, Papadogias D, Tsikini A, Makras P, Dimitriou K, Stathopoulou A, Piaditis G (2007) Polycystic ovaries and the polycystic ovary syndrome phenotype in women with active acromegaly. Clin Endocrinol 67(6):917–922. https://doi.org/10.1111/j.1365-2265.2007.02987.x
Maffei P, Tamagno G, Nardelli GB, Videau C, Menegazzo C, Milan G, Calcagno A, Martini C, Vettor R, Epelbaum J, Sicolo N (2010) Effects of octreotide exposure during pregnancy in acromegaly. Clin Endocrinol 72(5):668–677. https://doi.org/10.1111/j.1365-2265.2009.03706.x
Trainer PJ, Drake WM, Katznelson L, Freda PU, Herman-Bonert V, van der Lely AJ, Dimaraki EV, Stewart PM, Friend KE, Vance ML, Besser GM, Scarlett JA, Thorner MO, Parkinson C, Klibanski A, Powell JS, Barkan AL, Sheppard MC, Malsonado M, Rose DR, Clemmons DR, Johannsson G, Bengtsson BA, Stavrou S, Kleinberg DL, Cook DM, Phillips LS, Bidlingmaier M, Strasburger CJ, Hackett S, Zib K, Bennett WF, Davis RJ (2000) Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant. N Engl J Med 342(16):1171–1177. https://doi.org/10.1056/nejm200004203421604
van der Lely AJ, Hutson RK, Trainer PJ, Besser GM, Barkan AL, Katznelson L, Klibanski A, Herman-Bonert V, Melmed S, Vance ML, Freda PU, Stewart PM, Friend KE, Clemmons DR, Johannsson G, Stavrou S, Cook DM, Phillips LS, Strasburger CJ, Hackett S, Zib KA, Davis RJ, Scarlett JA, Thorner MO (2001) Long-term treatment of acromegaly with pegvisomant, a growth hormone receptor antagonist. Lancet (London, England) 358(9295):1754–1759. https://doi.org/10.1016/s0140-6736(01)06844-1
Buchfelder M, van der Lely AJ, Biller BMK, Webb SM, Brue T, Strasburger CJ, Ghigo E, Camacho-Hubner C, Pan K, Lavenberg J, Jonsson P, Hey-Hadavi JH (2018) Long-term treatment with pegvisomant: observations from 2090 acromegaly patients in ACROSTUDY. Eur J Endocrinol 179(6):419–427. https://doi.org/10.1530/eje-18-0616
Ghajar A, Jones P, Guarda FJ, Faje A, Tritos NA, Miller KK, Swearingen B, Nachtigall LB (2019) Biochemical control in acromegaly with multimodality therapies: outcomes from a pituitary center and changes over time. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgz187
Whittaker PG, Stewart MO, Taylor A, Howell RJ, Lind T (1990) Insulin-like growth factor 1 and its binding protein 1 during normal and diabetic pregnancies. Obstet Gynecol 76(2):223–229
Huang W, Molitch ME (2019) Pituitary Tumors in Pregnancy. Endocrinol Metab Clin North Am 48(3):569–581. https://doi.org/10.1016/j.ecl.2019.05.004
Chanson P, Vialon M, Caron P (2019) An update on clinical care for pregnant women with acromegaly. Expert Rev Endocrinol Metab 14(2):85–96. https://doi.org/10.1080/17446651.2019.1571909
Unuane D, Tournaye H, Velkeniers B, Poppe K (2011) Endocrine disorders & female infertility. Best practice & research. Clin Endocrinol Metab 25(6):861–873. https://doi.org/10.1016/j.beem.2011.08.001
Muhammad A, Neggers SJ, van der Lely AJ (2017) Pregnancy and acromegaly. Pituitary 20(1):179–184. https://doi.org/10.1007/s11102-016-0740-3
Leung KC, Johannsson G, Leong GM, Ho KK (2004) Estrogen regulation of growth hormone action. Endocr Rev 25(5):693–721. https://doi.org/10.1210/er.2003-0035
Leung KC, Doyle N, Ballesteros M, Sjogren K, Watts CK, Low TH, Leong GM, Ross RJ, Ho KK (2003) Estrogen inhibits GH signaling by suppressing GH-induced JAK2 phosphorylation, an effect mediated by SOCS-2. Proc Natl Acad Sci USA 100(3):1016–1021. https://doi.org/10.1073/pnas.0337600100
Hannon AM, O'Shea T, Thompson CA, Hannon MJ, Dineen R, Khattak A, Gibney J, O'Halloran DJ, Hunter S, Thompson CJ, Sherlock M (2019) Pregnancy in acromegaly is safe and is associated with improvements in IGF-1 concentrations. Eur J Endocrinol 180(4):K21–k29. https://doi.org/10.1530/eje-18-0688
Caron P, Broussaud S, Bertherat J, Borson-Chazot F, Brue T, Cortet-Rudelli C, Chanson P (2010) Acromegaly and pregnancy: a retrospective multicenter study of 59 pregnancies in 46 women. J Clin Endocrinol Metab 95(10):4680–4687. https://doi.org/10.1210/jc.2009-2331
Jallad RS, Shimon I, Fraenkel M, Medvedovsky V, Akirov A, Duarte FH, Bronstein MD (2018) Outcome of pregnancies in a large cohort of women with acromegaly. Clin Endocrinol 88(6):896–907. https://doi.org/10.1111/cen.13599
Vialon M, Grunenwald S, Mouly C, Vezzosi D, Bennet A, Gourdy P, Caron PJ (2019) Gestational diabetes and acromegaly: single-centre experience of 14 pregnancies. Clin Endocrinol 91(6):805–809. https://doi.org/10.1111/cen.14097
Haliloglu O, Dogangun B, Ozcabi B, Kural HU, Keskin FE, Ozkaya HM, Pamukcu FC, Bektas E, Poyraz BC, Buber H, Evliyaoglu O, Kadioglu P (2016) General health status and intelligence scores of children of mothers with acromegaly do not differ from those of healthy mothers. Pituitary 19(4):391–398. https://doi.org/10.1007/s11102-016-0717-2
Dias M, Boguszewski C, Gadelha M, Kasuki L, Musolino N, Vieira JG, Abucham J (2014) Acromegaly and pregnancy: a prospective study. Eur J Endocrinol 170(2):301–310. https://doi.org/10.1530/eje-13-0460
Cheng S, Grasso L, Martinez-Orozco JA, Al-Agha R, Pivonello R, Colao A, Ezzat S (2012) Pregnancy in acromegaly: experience from two referral centers and systematic review of the literature. Clin Endocrinol 76(2):264–271. https://doi.org/10.1111/j.1365-2265.2011.04180.x
Katznelson L, Laws ER Jr, Melmed S, Molitch ME, Murad MH, Utz A, Wass JA (2014) Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 99(11):3933–3951. https://doi.org/10.1210/jc.2014-2700
Tritos NA, Biller BM (2017) Pegvisomant: a growth hormone receptor antagonist used in the treatment of acromegaly. Pituitary 20(1):129–135. https://doi.org/10.1007/s11102-016-0753-y
Brooks AJ, Waters MJ (2010) The growth hormone receptor: mechanism of activation and clinical implications. Nat Rev Endocrinol 6(9):515–525. https://doi.org/10.1038/nrendo.2010.123
van der Lely AJ, Gomez R, Heissler JF, Akerblad AC, Jonsson P, Camacho-Hubner C, Koltowska-Haggstrom M (2015) Pregnancy in acromegaly patients treated with pegvisomant. Endocrine 49(3):769–773. https://doi.org/10.1007/s12020-014-0508-3
Qureshi A, Kalu E, Ramanathan G, Bano G, Croucher C, Panahloo A (2006) IVF/ICSI in a woman with active acromegaly: successful outcome following treatment with pegvisomant. J Assist Reprod Genet 23(11–12):439–442. https://doi.org/10.1007/s10815-006-9077-6
Brian SR, Bidlingmaier M, Wajnrajch MP, Weinzimer SA, Inzucchi SE (2007) Treatment of acromegaly with pegvisomant during pregnancy: maternal and fetal effects. J Clin Endocrinol Metab 92(9):3374–3377. https://doi.org/10.1210/jc.2007-0997
Funding
None.
Author information
Authors and Affiliations
Contributions
LBN conceived of and directed the study and provided clinical information on case 1. MG provided clinical information on cases 2 and 3. WG and FJG extracted the clinical data, performed the literature search and drafted the manuscript. WG, FJG, AG, MG and LBN collectively revised the manuscript and approved its final form.
Corresponding author
Ethics declarations
Conflict of interest
WG, FJG and AG: none. MG received honoraria for consulting from Pfizer and Sanofi-Aventis and from Novartis for an ongoing trial. LBN received honoraria for consulting from Pfizer and Ipsen, and research funds from Ipsen and Chiasma.
Ethical approval
The study was approved by the Institutional Review Board of Partners Healthcare.
Consent to participate
Not applicable for retrospective observational study.
Consent for publication
Not applicable for retrospective observational study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guarda, F.J., Gong, W., Ghajar, A. et al. Preconception use of pegvisomant alone or as combination therapy for acromegaly: a case series and review of the literature. Pituitary 23, 498–506 (2020). https://doi.org/10.1007/s11102-020-01050-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-020-01050-2