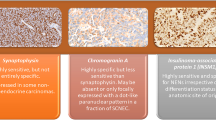
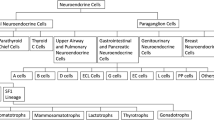
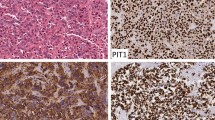
Abstract
Purpose
Pituitary tumor is the common primary brain tumor in humans. For further studying the pathogenesis and new therapeutic targets of pituitary adenoma, cell lines and primary cells are necessary tools. Different from primary cells that have short survival time and hormone secretion maintenance time, cell lines would be endowed with immortal characteristics under the help of gene modification. This review is to explore whether these cell lines still have similar pathophysiological changes in pituitary adenoma cells and methods to prolong the lifespan of pituitary adenoma primary cells.
Results
In the cell lines summarized in the review, HP75, PDFS, HPA and GX were derived from human pituitary adenomas. It was found that the cell lines commonly used in articles published between January 2014 and July 2019 were GH3, AtT20, MMQ, GH4C1, HP75 and TtT/GF. Besides, it was glad that many methods had been used to prolong the lifespan and maintain characteristics of pituitary adenoma primary cells.
Conclusion
The paper reviews most of pituitary adenoma cell lines that have been successfully established since 1968 and the relevant situation of primary culture of pituitary adenoma cells. Obviously, it requires us to make more efforts to obtain human pituitary adenoma cell lines and prolong the lifespan of pituitary adenoma primary cells with maintaining their morphology and ability to secret hormones.
Similar content being viewed by others
References
Pennacchietti V, Garzaro M, Grottoli S, Pacca P, Garbossa D, Ducati A, Zenga F (2016) Three-dimensional endoscopic endonasal approach and outcomes in sellar lesions: a single-center experience of 104 cases. World Neurosurg 89:121–125
Laws ER, Penn DL, Repetti CS (2019) Advances and controversies in the classification and grading of pituitary tumors. J Endocrinol Invest 42(2):129–135
Lim CT, Korbonits M (2018) Update on the clinicopathology of pituitary adenomas. Endocr Practice Off J Am Coll Endocrinol Am Assoc Clin Endocrinol 24(5):473–488
Molitch ME (2017) Diagnosis and treatment of pituitary adenomas: a review. JAMA 317(5):516–524
Feng YJ, Mao ZG, Wang X, Du Q, Jian MY, Zhu DM, Xiao Z, Wang HJ, Zhu YH (2018) MicroRNAs and target genes in pituitary adenomas. Horm Metab Res 50(3):e3
Aflorei ED, Korbonits M (2014) Epidemiology and etiopathogenesis of pituitary adenomas. J Neurooncol 117(3):379–394
Lloyd RV, Osamura RY, Klöppel G, Rosai J (2017) WHO classifcation of tumours of endocrine organs, 4th edn. IARC Press, Lyon
Tashjian AH, Yasumura Y, Levine L, Sato GH, Parker ML (1968) Establishment of clonal strains of rat pituitary tumor cells that secrete growth hormone. Endocrinology 82(2):342–352
Yasumura Y (1968) Retention of differentiated function in clonal animal cell lines, particularly hormone-secreting cultures. Am Zool 8(2):285–305
Sonnenschein C, Richardson UI, Tashjian AH (1970) Chromosomal analysis, organ-specific function and appearance of six clonal strains of rat pituitary tumor cells. Exp Cell Res 61(1):121–128
Tashjian AH, Bancroft FC, Levine L (1970) Production of both prolactin and growth hormone by clonal strains of rat pituitary tumor cells. Differential effects of hydrocortisone and tissue extracts. J Cell Biol 47(1):61–70
Richardson UI (1976) Establishment in culture of a multihormone-secreting cell strain derived from the MtT/F4 rat pituitary tumor. J Cell Physiol 88(3):287–296
Yu LY, Tushinski RJ, Bancroft FC (1977) Glucocorticoid induction of growth hormone synthesis in a strain of rat pituitary cells. J Biol Chem 252(11):3870
Tashjian AH (1979) Clonal strains of hormone-producing pituitary cells. Methods Enzymol 58:527–535
Reymond MJ, Nansel DD, Burrows GH, Neaves WB, Porter JC (1984) A new clonal strain of rat pituitary tumour cells: a model for non-regulated secretion of prolactin. Acta Endocrinol 106(4):459–470
Kikuchi Y, Seki K, Momose E, Kizawa I, Oomori K, Shima K, Mukai K, Kato K (1985) Establishment and characterization of a new human cultured cell line from a prolactin-secreting pituitary adenoma. Can Res 45(11 Pt 2):5722–5727
Judd AM, Login IS, Kovacs K, Ross PC, Spangelo BL, Jarvis WD, Macleod RM (1988) Characterization of the MMQ cell, a prolactin-secreting clonal cell line that is responsive to dopamine. Endocrinology 123(5):2341
Hurbain-Kosmath I, Berault A, Noel N, Polkowska J, Bohin A, Jutisz M, Leiter EH, Beamer WG, Bedigian HG, Davisson MT (1990) Gonadotropes in a novel rat pituitary tumor cell line, RC-4B/C. Establishment and partial characterization of the cell line. Vitro Cell Dev Biol 26(5):431–440
Inoue K, Hattori M, Sakai T, Inukai S, Fujimoto N, Ito A (1990) Establishment of a series of pituitary clonal cell lines differing in morphology, hormone secretion, and response to estrogen. Endocrinology 126(5):2313–2320
Windle JJ, Weiner RI, Mellon PL (1990) Cell lines of the pituitary gonadotrope lineage derived by targeted oncogenesis in transgenic mice. Mol Endocrinol 4(4):597–603
Ivins KJ, Molinoff PB (1990) Serotonin-2 receptors coupled to phosphoinositide hydrolysis in a clonal cell line. Mol Pharmacol 37(5):622–630
Ivins KJ, Molinoff PB (1990) Receptors for monoamines on cultured cells. Am J Hypertens 3(6 Pt 2):43S–46S
Akerblom IE, Ridgway EC, Mellon PL (1990) An α-subunit-secreting cell line derived from a mouse thyrotrope tumor. Mol Endocrinol 4(4):589–596
Inoue K, Matsumoto H, Koyama C, Shibata K, Nakazato Y, Ito A (1992) Establishment of a folliculo-stellate-like cell line from a murine thyrotropic pituitary tumor. Endocrinology 131(6):3110–3116
Chomczynski P, Soszynski PA, Frohman LA (1993) Stimulatory effect of thyroid hormone on growth hormone gene expression in a human pituitary cell line. J Clin Endocrinol Metabol 77(1):281–285
Alarid ET, Windle JJ, Whyte DB, Mellon PL (1996) Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development 122(10):3319
Graham KE, Nusser KD, Low MJ (1999) LbetaT2 gonadotroph cells secrete follicle stimulating hormone (FSH) in response to active A. J Endocrinol 162(3):1–5
Aoki Y, Iwasaki Y, Katahira M, Oiso Y, Saito H (1997) Regulation of the rat proopiomelanocortin gene expression in AtT-20 cells. I: effects of the common secretagogues. Endocrinology 138(5):1923
Jin L, Kulig E, Qian X, Scheithauer BW, Eberhardt NL, Lloyd RV (1998) A human pituitary adenoma cell line proliferates and maintains some differentiated functions following expression of SV40 large T-antigen. Endocr Pathol 9(2):169–184. https://doi.org/10.1007/bf02782609
Danila DC, Zhang X, Zhou Y, Dickersin GR, Fletcher JA, Hedleywhyte ET, Selig MK, Johnson SR, Klibanski A (2000) A human pituitary tumor-derived folliculostellate cell line. J Clin Endocrinol Metab 85(3):1180
Ishikawa H (1969) Isolation of different types of anterior pituitary cells in rats. Endocrinol Jpn 16(5):517–529
Chatterjee P (1976) The effect of culture on ultrastructure of dissociated rabbit adenohypophysial cells. J Anat 121(Pt 2):241–258
Allen RG, Herbert E, Hinman M, Shibuya H, Pert CB (1978) Coordinate control of corticotropin, β-lipotropin, and β-endorphin release in mouse pituitary cell cultures. Proc Natl Acad Sci USA 75(10):4972–4976
Lin SW, Ge W (2009) Differential regulation of gonadotropins (FSH and LH) and growth hormone (GH) by neuroendocrine, endocrine, and paracrine factors in the zebrafish–an in vitro approach. Gen Comp Endocrinol 160(2):183–193
Ellis MJ, Mulligan RS, Evans MJ, Donald RA (1994) The effects of corticotrophin-releasing hormone, arginine vasopressin and their antagonists on ACTH release from perifused horse anterior pituitary cells. J Endocrinol 143(1):85–93
Kemppainen RJ, Zerbe CA, Sartin JL (1989) Regulation and secretion of proopiomelanocortin peptides from isolated perifused dog pituitary pars intermedia cells. Endocrinology 124(5):2208
Haug TM, Kjetil H, Finn-Arne W, Olav S (2007) Electrophysiological properties of pituitary cells in primary culture from Atlantic cod (Gadus morhua). Neuroendocrinology 86(1):38–47
Chen D, Yang W, Han S, Yang H, Cen X, Liu J, Zhang L, Zhang W (2018) A type IIb, but not type IIa, GnRH receptor mediates GnRH-induced release of growth hormone in the Ricefield Eel. Front Endocrinol 9:721
Campo A, Lafont AG, Lefranc B, Leprince J, Tostivint H, Kamech N, Dufour S, Rousseau K (2018) Tachykinin-3 genes and peptides characterized in a basal teleost, the European eel: evolutionary perspective and pituitary role. Front Endocrinol 9:304
Chen LR, Lee SC, Lin YP, Hsieh YL, Chen YL, Yang JR, Liou JF, Chen CF, Lee YP, Shiue YL (2010) Prostaglandin-D synthetase induces transcription of the LH beta subunit in the primary culture of chicken anterior pituitary cells via the PPAR signaling pathway. Theriogenology 73(3):367–382
Kineman RD, Luque RM (2007) Evidence that ghrelin is as potent as growth hormone (GH)-releasing hormone (GHRH) in releasing GH from primary pituitary cell cultures of a nonhuman primate (Papio anubis), acting through intracellular signaling pathways distinct from GHRH. Endocrinology 148(9):4440–4449
Melmed S, Odenheimer D, Carlson HE, Hershman JM (1982) Establishment of functional human pituitary tumor cell cultures. Vitro 18(1):35–42
Ishibashi M, Yamaji T (1984) Direct effects of catecholamines, thyrotropin-releasing hormone, and somatostatin on growth hormone and prolactin secretion from adenomatous and nonadenomatous human pituitary cells in culture. J Clin Investig 73(1):66–78
Ishiwata I, Ishiwata C, Iguchi M, Soma M, Sato Y, Sonobe M, Kiguchi K, Tachibana T, Ishikawa H (2004) Biological characteristics of cultured cells derived from various types of human brain tumors. Hum Cell 17(3):117–124
Fazekas I, Hegedüs B, Bácsy E, Kerekes E, Slowik F, Balint K, Pásztor E (2005) Characterization of human pituitary adenomas in cell cultures by light and electron microscopic morphology and immunolabeling. Folia Histochem Cytobiol 43(2):81–90
Yoshida T, Hayakawa T, Mori S, Ushio Y, Nakagawa H, Mogami H, Nakata Y (1983) Primary culture of human functioning pituitary adenoma in monolayer and collagen gel culture. No Shinkei Geka Neurol Surg 11(11):1149–1155
Avila-RodrãGuez D, Paisano-Cerã NK, Valdovinos-RamãRez I, Solano-Agama C, Ortiz-Plata A., Mendoza-Garrido ME (2016) Three-dimensional alginate-bead culture of human pituitary adenoma cells. J Vis Exp 108: 53637
Aiello A, Cassarino MF, Nanni S, Sesta A, Ferraú F, Grassi C, Losa M, Trimarchi F, Pontecorvi A, Cannavò S (2018) Establishment of a protocol to extend the lifespan of human hormone-secreting pituitary adenoma cells. Endocrine 59(1):102–108
Perrone MH, Greer TL, Hinkle PM (1980) Relationships between thyroid hormone and glucocorticoid effects in GH3 pituitary cells. Endocrinology 106(2):600–605
Hashimoto S, Yoshimura H, Okada K, Uramaru N, Sugihara K, Kitamura S, Imaoka S (2012) Effects of polybrominated diphenyl ethers (PBDEs) and their derivatives on protein disulfide isomerase activity and growth hormone release of GH3 cells. Chem Res Toxicol 25(3):656–663
Shahmoon S, Rubinfeld H, Wolf I, Cohen ZR, Hadani M, Shimon I, Rubinek T (2014) The aging suppressor klotho: a potential regulator of growth hormone secretion. Am J Physiol 307(3):E326–E334
de Dios N, Orrillo S, Irizarri M, Theas MS, Boutillon F, Candolfi M, Seilicovich A, Goffin V, Pisera D, Ferraris J (2019) JAK2/STAT5 pathway mediates prolactin-induced apoptosis of lactotropes. Neuroendocrinology 108(2):84–97
Peverelli E, Giardino E, Treppiedi D, Catalano R, Mangili F, Locatelli M, Lania AG, Arosio M, Spada A, Mantovani G (2018) A novel pathway activated by somatostatin receptor type 2 (SST2): inhibition of pituitary tumor cell migration and invasion through cytoskeleton protein recruitment. Int J Cancer 142(9):1842–1852
Richardson UI, Schonbrunn A (1981) Inhibition of adrenocorticotropin secretion by somatostatin in pituitary cells in culture. Endocrinology 108(1):281
Jian F, Chen Y, Ning G, Fu W, Tang H, Chen X, Zhao Y, Zheng L, Pan S, Wang W, Bian L, Sun Q (2016) Cold inducible RNA binding protein upregulation in pituitary corticotroph adenoma induces corticotroph cell proliferation via Erk signaling pathway. Oncotarget 7(8):9175–9187
Parvin R, Noro E, Saitohakoda A, Shimada H, Suzuki S, Shimizu K, Miyachi H, Yokoyama A, Sugawara A (2018) Inhibitory effects of a novel PPAR-γ agonist MEKT1 on Pomc expression/ACTH secretion in AtT20 cells. PPAR Res 2018:5346272
Xu Y, Wang Y, Ma G, Wang Q, Wei G (2014) CUL4A is overexpressed in human pituitary adenomas and regulates pituitary tumor cell proliferation. J Neurooncol 116(3):625–632
Li R, Zhang Z, Wang J, Huang Y, Sun W, Xie R, Hu F, Lei T (2017) Triptolide suppresses growth and hormone secretion in murine pituitary corticotroph tumor cells via NF-kappaB signaling pathway. Biomed Pharmacother 95:771–779
Polidoro MA, Rotondi S, Morace R, Rostomyan L, Colapietro A, Arcella A, Ventura L, Angelucci A, Giangaspero F, Esposito V, Beckers A, Jaffrain-Rea ML (2018) Expression of peroxisome proliferator-activated receptor alpha (PPARα) in non-somatotroph pituitary tumours and the effects of PPARα agonists on MMQ cells. Horm Metab Res 50(8):640–647
Gangisetty O, Jabbar S, Wynne O, Sarkar DK (2017) MicroRNA-9 regulates fetal alcohol induced changes in D2 receptor to promote prolactin production. J Endocrinol 235(1):1–14
Lin SJ, Wu ZR, Cao L, Zhang Y, Leng ZG, Guo YH, Shang HB, Zhao WG, Zhang X, Wu ZB (2017) Pituitary tumor suppression by combination of cabergoline and chloroquine. J Clin Endocrinol Metab 102(10):3692
Wei Y, Zhou X, Ren L, Wang C, Li Y (2018) The prolactin-release inhibitor paeoniflorin suppresses proliferation and induces apoptosis in prolactinoma cells via the mitochondria-dependent pathway. J Cell Biochem 119(7):5704–5714
Cui M, Zhang M, Liu HF, Wang JP (2017) Effects of microRNA-21 targeting PITX2 on proliferation and apoptosis of pituitary tumor cells. Eur Rev Med Pharmacol Sci 21(13):2995–3004
Wang DW, Wang YQ, Shu HS (2018) MiR-16 inhibits pituitary adenoma cell proliferation via the suppression of ERK/MAPK signal pathway. Eur Rev Med Pharmacol Sci 22(5):1241–1248
Peverelli E, Giardino E, Treppiedi D, Locatelli M, Vaira V, Ferrero S, Bosari S, Lania AG, Spada A, Mantovani G (2016) Dopamine receptor type 2 (DRD2) inhibits migration and invasion of human tumorous pituitary cells through ROCK-mediated cofilin inactivation. Cancer Lett 381(2):279–286
Wang J, Voellger B, Benzel J, Schlomann U, Nimsky C, Bartsch JW, Carl B (2016) Metalloproteinases ADAM12 and MMP-14 are associated with cavernous sinus invasion in pituitary adenomas. Int J Cancer 139(6):1327–1339
Voellger B, Waldt N, Rupa R, Kirches E, Melhem O, Ochel HJ, Mawrin C, Firsching R (2018) Combined effects of resveratrol and radiation in GH3 and TtT/GF pituitary adenoma cells. J Neurooncol 139(2–3):1–10
Koike K, Zhen XZ, Sakamoto Y, Kanda Y, Murakami K, Miyake A, Inoue M (1997) The pituitary folliculo-stellate cell line TtT/GF augments basal and trhinduced prolactin secretion by GH3 cell. Life Sci 61(25):2491
Brokken LJ, Leendertse M, Bakker O, Wiersinga WM, Prummel MF (2004) Expression of adenohypophyseal-hormone receptors in a murine folliculo-stellate cell line. Horm Metab Res 36(08):538–541
Tsukada T, Yoshida S, Kito K, Fujiwara K, Yako H, Horiguchi K, Isowa Y, Yashiro T, Kato T, Kato Y (2018) TGFβ signaling reinforces pericyte properties of the non-endocrine mouse pituitary cell line TtT/GF. Cell Tissue Res 371(2):339–350
Osborne R, Tashjian AH (1981) Tumor-promoting phorbol esters affect production of prolactin and growth hormone by rat pituitary cells. Endocrinology 108(4):1164
Haug E, Naess O, Gautvik KM (1978) Receptors for 17beta-estradiol in prolactin-secreting rat pituitary cells. Mol Cell Endocrinol 12(1):81–95
Koenig RJ, Warne RL, Brent GA, Harney JW, Larsen PR, Moore DD (1988) Isolation of a cDNA clone encoding a biologically active thyroid hormone receptor. Proc Natl Acad Sci USA 85(14):5031–5035
Patel YC, Murthy KK, Escher EE, Banville D, Spiess J, Srikant CB (1990) Mechanism of action of somatostatin: an overview of receptor function and studies of the molecular characterization and purification of somatostatin receptor proteins. Metab Clin Exp 39(9):63–69
Tani Y, Yamada S, Inoshita N, Hirata Y, Shichiri M (2015) Regulation of growth hormone secretion by (pro)renin receptor. Sci Rep 5:10878
Cronin MJ, Faure N, Martial JA, Weiner RI (1980) Absence of high affinity dopamine receptor in GH3 cells: a prolactin-secreting clone resistant to the inhibitory action of dopamine. Endocrinology 106(3):718
Funding
This study was funded by Research Project of Traditional Chinese Medicine Bureau of Guangdong Province (Grant No. 20173003).
Author information
Authors and Affiliations
Contributions
ZZ and WC have contributed equally to this work. ZZ and WC drafted the manuscript and performed the literature search. DZ and NG performed the literature search. YZ had the idea for the article and critically revised the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, Z., Cui, W., Zhu, D. et al. Common tools for pituitary adenomas research: cell lines and primary cells. Pituitary 23, 182–188 (2020). https://doi.org/10.1007/s11102-019-01003-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-019-01003-4