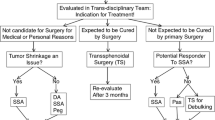
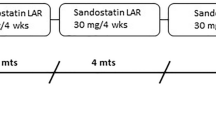
Abstract
Purpose
To investigate the effects of preoperative somatostatin analogue (SSA) treatment on the annual cost of all acromegaly treatment modalities and on remission rates.
Methods
The medical records of 135 patients with acromegaly who were followed at endocrinology clinic of Cerrahpasa Medical Faculty for at least 2 years after surgery between 2009 and 2016 were reviewed.
Results
The mean follow-up time was 50.9 ± 25.7 months. Early remission was defined according to 3rd month values in patients who didn’t achieve remission, and 6th month values in patients who achieved remission at the 3rd month after surgery. The early and late remission rates of the entire study population were 40% and 80.7%, respectively. The early remission of the preoperative SSA-treated group (61.5%) was significantly higher than SSA-untreated group (31.2%) (p = 0.002). The early remission of the preoperative SSA-treated patients with macroadenomas (52.2%) was also significantly higher than the SSA-untreated group (23.5%) (p = 0.02). In the subgroup analysis; this difference was much more pronounced in invasive macroadenomas (p = 0.002). There were no differences between the groups in terms of late remission.The median annual cost of all acromegaly treatment modalities in study population was €3788.4; the cost for macroadenomas was significantly higher than for microadenomas (€4125.0 vs. €3226.5, respectively; p = 0.03). Preoperative SSA use in both microadenomas and macroadenomas didn’t alter the cost of treatment. The increase in the duration of preoperative medical treatment had no effect on early or late remissions (p = 0.09; p = 0.8).
Conclusions
Preoperative medical treatment had no effect on the costs of acromegaly treatment. There was a benefical effect of pre-operative SSA use on early remission in patients with macroadenomas; however, this effect didn’t persist long term.


Similar content being viewed by others
References
Katznelson L, Laws ER Jr, Melmed S, Molitch ME, Murad MH, Utz A, Wass JAH (2014) Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 99:3933–3951
Wilson LS, Shin JL, Ezzat S (2001) Longitudinal assessment of economic burden and clinical outcomes in acromegaly. Endocr Pract 7:170–180
Katznelson L, Atkinson JL, Cook DM, Ezzat SZ, Hamrahian AH, Miller KK (2011) American Association of Clinical Endocrinologists medical guidelines for clinical practice forthe diagnosis and treatment of acromegaly–2011 update. Endocr Pract 4:1–44
Ben-Shlomo A, Sheppard MC, Stephens JM, Pulgar S, Melmed S (2011) Clinical, quality of life, and economic value of acromegaly disease control. Pituitary 14:284–294
Abu Dabrh AM, Mohammed K, Asi N, Farah WH, Wang Z, Farah MH, Prokop LJ, Katznelson L, Murad MH (2014) Surgical interventions and medical treatments in treatment-naive patients with acromegaly: systematic review and meta-analysis. J Clin Endocrinol Metab 99:4003–4014
Melmed S, Colao A, Barkan A, Molitch M, Grossman AB, Kleinberg D, Clemmons D, Chanson P, Laws E, Schlechte J, Vance ML, Ho K, Giustina A, Acromegaly Consensus Group (2009) Guidelines for acromegaly management: an update. J Clin Endocrinol Metab 94:1509–1517
Jane JA Jr, Starke RM, Elzoghby MA, Reames DL, Payne SC, Thorner MO, Marshall JC, Laws ER Jr, Vance ML (2011) Endoscopic transsphenoidal surgery for acromegaly: remission using modern criteria, complications, and predictors of outcome. J Clin Endocrinol Metab 96:2732–2740
Starke RM, Raper DM, Payne SC, Vance ML, Oldfield EH, Jane JA Jr (2013) Endoscopic vs microsurgical transsphenoidal surgery for acromegaly: outcomes in a concurrent series of patients using modern criteria for remission. J Clin Endocrinol Metab 98:3190–3198
Haliloglu O, Kuruoglu E, Ozkaya HM, Keskin FE, Gunaldi O, Oz B, Gazioglu N, Kadioglu P, Tanriover N (2016) Multidisciplinary approach for acromegaly: a single tertiary Center’s experience. World Neurosurg 88:270–276
Nunes VS, Correa JM, Puga ME, Silva EM, Boguszewski CL (2015) Preoperative somatostatin analogues versus direct transsphenoidal surgery for newly-diagnosed acromegaly patients: a systematic review and meta-analysis using the GRADE system. Pituitary 18:500–508
Abe T, Ludecke DK (2001) Effects of preoperative octreotide treatment on different subtypes of 90 GH-secreting pituitary adenomas and outcome in one surgical centre. Eur J Endocrinol 145:137–145
Carlsen SM, Lund-Johansen M, Schreiner T, Aanderud S, Johannesen O, Svartberg J, Cooper JG, Hald JK, Fougner SL, Bollerslev J, Preoperative Octreotide Treatment of Acromegaly study group (2008) Preoperative octreotide treatment in newly diagnosed acromegalic patients with macroadenomas increases cure short-term postoperative rates: a prospective, randomized trial. J Clin Endocrinol Metab 93:2984–2990
Stevenaert A, Beckers A (1993) Presurgical octreotide treatment in acromegaly. Acta Endocrinol 129:18–20
Kristof RA, Stoffel-Wagner B, Klingmuller D, Schramm J (1999) Does octreotide treatment improve the surgical results of macro-adenomas in acromegaly? A randomized study. Acta Neurochir 141:399–405
Losa M, Mortini P, Urbaz L, Ribotto P, Castrignanó T, Giovanelli M (2006) Presurgical treatment with somatostatin analogs in patients with acromegaly: effects on the remission and complication rates. J Neurosurg 104:899–906
Plockinger U, Quabbe HJ (2005) Presurgical octreotide treatment in acromegaly: no improvement of final growth hormone (GH) concentration and pituitary function. A long-term case-control study. Acta Neurochir 147:485–493
Shen M, Shou X, Wang Y, Zhang Z, Wu J, Mao Y, Li S, Zhao Y (2010) Effect of presurgical long-acting octreotide treatment in acromegaly patients with invasive pituitary macroadenomas: a prospective randomized study. Endocr J 57:1035–1044
Li Z-Q, Quan Z, Tian H-L, Cheng M (2012) Preoperative lanreotide treatment improves outcome in patients with acromegaly resulting from invasive pituitary macroadenoma. J IntMedRes 40:517–524
Mao ZG, Zhu YH, Tang HL, Wang DY, Zhou J, He DS, Lan H, Luo BN, Wang HJ (2010) Preoperative lanreotide treatment in acromegalic patients with macroadenomas increases short-term postoperative cure rates: a prospective, randomised trial. Eur J Endocrinol 162:661–666
Colao A, Auriemma RS, Lombardi G, Pivonello R (2011) Resistance to somatostatin analogs in acromegaly. Endocrine Rev 32:247–271
Caron PJ, Bevan JS, Petersenn S, Flanagan D, Tabarin A, Prévost G, Maisonobe P, Clermont A (2014) Tumor shrinkage with lanreotide Autogel 120 mg as primary therapy in acromegaly: results of a prospective multicenter clinical trial. J Clin Endocrinol Metab 99:1282–1290
Melmed S, Sternberg R, Cook D, Klibanski A, Chanson P, Bonert V, Vance ML, Rhew D, Kleinberg D, Barkan A (2005) A critical analysis of pituitary tumor shrinkage during primary medical therapy in acromegaly. J Clin Endocrinol Metab 90:4405–4410
Jacob JJ, Bevan JS (2014) Should all patients with acromegaly receive somatostatin analogue therapy before surgery and if so, for how long? Clin Endocrinol 81:812–817
Fleseriu M, Hoffman AR, Katznelson L (2015) AACE neuroendocrine and pituitary Scientific Committee. American Association of Clinical Endocrinologists and Amerıcan College of Endocrınology Disease State Clinical Review: management of acromegaly patients: what is the role of pre-operative medical therapy? Endocr Pract 21:668–673
Zhang L, Wu X, Yan Y, Qian J, Lu Y, Luo C (2015) Preoperative somatostatin analogs treatment in acromegalic patients with macroadenomas. A meta-analysis. Brain Dev 37:181–190
Beckers A (2008) Does preoperative somatostatin analog treatment improve surgical cure rates in acromegaly? A new look at an old question. J Clin Endocrinol Metab 93:2975–2977
Fougner SL, Bollerslev J, Svartberg J, Oksnes M, Cooper J, Carlsen SM (2014) Preoperative octreotide treatment of acromegaly: long-term results of a randomised controlled trial. Eur J Endocrinol 171:229–235
Margusino-Framinan L, Pertega-Diaz S, Pena-Bello L, Sangiao-Alvarellos S, Outeirino-Blanco E, Pita-Gutierrez F, Cordido F (2015) Cost-effectivenes analysis of preoperative treatment of acromegalywith somatostatin analogue on surgical outcome. Eur J Int Med 26:736–741
Duan L, Huang M, Yan H, Zhang Y, Gu F (2015) Cost-effectiveness analysis of two therapeutic schemes in the treatment of acromegaly: a retrospective study of 168 cases. J Endocrinol Invest 38:717–723
Knosp E, Steiner E, Kitz K, Matula C (1993) Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 33:610–617
Duan L, Zhu H, Xing B, Gu F (2017) Prolonged preoperativetreatment of acromegaly with Somatostatin analogs may improve surgical outcome in patients with invasive pituitary macroadenoma (Knosp grades 1-3): a retrospective cohort study conducted at a single center. BMC Endocr Disord 17:55
Fleseriu M (2011) Clinical efficacy and safety results for dose escalation of somatostatin receptor ligands in patients with acromegaly: a literature review. Pituitary 14:184–193
Losa M, Ciccarelli E, Mortini P, Barzaghi R, Gaia D, Faccani G, Papotti M, Mangili F, Terreni MR, Camanni F, Giovanelli M (2001) Effects of octreotide treatment on the proliferation and apoptotic index of GH-secreting pituitary adenomas. J Clin Endocrinol Metab 86:5194–5200
Cap J, Cerman J, Nemecek S, Marekova M, Hana V, Frysak Z (2003) The influence of treatment with somatostatin analogues on morphology, proliferative and apoptotic activity in GH-secreting pituitary adenomas. J Clin Neurosci 10(4):444–448
Roset M, Merino-Montero S, Luque-Ramírez M, Webb SM, López-Mondéjar P, Salinas I, Soto A, Bernal C, Villabona C, De Luis D, Donnay S, Pascual H, Pérez-Luis J, Spanish group of the OASIS study (2012) Cost of clinical management of acromegaly in Spain. Clin Drug Investig 32:235–245
Didoni G, Grottol S, Gasco V, Battistini M, Ferone D, Giusti M, Ragazzoni F, Ruffo P, Ghigo E, Minuto F (2004) Cost-of-illness study in acromegalic patients in Italy. J Endocrinol Invest 27:1034–1039
Moore DJ, Adi Y, Connock MJ, Bayliss S (2009) Clinical effectiveness and cost effectiveness of pegvisomant for the treatment of acromegaly: a systematic review and economic evaluation. BMC Endocr Disord 8:9–20
Acknowledgments
This research did not receive any specific grant from any funding agencies in the public, commercial, or non-profit sector. We would like to thank David Chapman for his help with the proofreading this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the local ethics committee of Istanbul University-Cerrahpasa, Cerrahpasa Medical Faculty and all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Research involving human and animal participants
This article does not contain any studies with animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Polat Korkmaz, O., Gurcan, M., Nuhoglu Kantarci, F.E. et al. The effects of pre-operative somatostatin analogue therapy on treatment cost and remission in acromegaly. Pituitary 22, 387–396 (2019). https://doi.org/10.1007/s11102-019-00968-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-019-00968-6