Abstract
Purpose
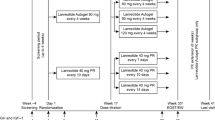
This analysis evaluates the 2-year effectiveness and safety of lanreotide depot/autogel (LAN), as well as treatment convenience and acromegaly symptom relief, from the Somatuline® Depot for Acromegaly (SODA) registry, a post-marketing, open-label, observational, multicenter, United States registry study.
Methods
Patients with acromegaly treated with LAN were eligible for enrollment. Demographics, LAN dose, extended dosing interval (EDI) (interval of injections ≥42 days), insulin-like growth factor 1 (IGF-1), growth hormone (GH), glycated hemoglobin, adverse events (AEs), injection convenience, and symptom data were collected.
Results
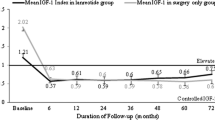
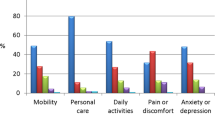
As of September 29, 2014, 241 patients were enrolled in SODA. IGF-1 levels below age- and gender-adjusted upper normal limit (ULN) were achieved in 71.2% at month (M) 12 and 74.4% at M24; GH ≤2.5 µg/L in 83.3% at M12 and 80.0% at M24; GH <1.0 µg/L in 61.7% at M12 and 61.4% at M24. Both IGF-1 < ULN and GH ≤2.5 µg/L were achieved in 65.0% at M12 and 54.8% at M24; both IGF-1 < ULN and GH < 1.0 µg/L were achieved in 51.7 and 42.9% at M12 and M24, respectively. EDI regimen was 5.0% at baseline and 12.0% at M24. At M24, acromegaly symptoms appeared stable or improved. The most common AE was arthralgia (25.7%). Among 106 serious AEs reported by 42 patients, 10 were deemed related to therapy in 9 patients. At M24, 73.1% of patients rated LAN as convenient.
Conclusions
SODA indicates 2-year biochemical control with majority of patients achieving both IGF-1 < ULN and GH ≤2.5 µg/L. LAN was generally well tolerated with no new or unexpected safety signals reported during the observation period.
clinicaltrials.gov Clinical Trial Identifier: NCT00686348




Similar content being viewed by others
References
Melmed S, Casanueva FF, Klibanski A, Bronstein MD, Chanson P, Lamberts SW, Strasburger CJ, Wass JAH, Giustina A (2013) A consensus on the diagnosis and treatment of acromegaly complications. Pituitary 16:294–302
Katznelson L, Laws ER Jr, Melmed S, Molitch ME, Murad MH, Utz A, Wass JA (2014) Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 99:3933–3951
Melmed S, Colao A, Barkan A, Molitch M, Grossman AB, Kleinberg D, Clemmons D, Chanson P, Laws E, Schlechte J, Vance ML, Ho K, Giustina A (2009) Guidelines for acromegaly management: an update. J Clin Endocrinol Metab 94:1509–1517
Giustina A, Chanson P, Bronstein MD, Klibanski A, Lamberts S, Casanueva FF, Trainer P, Ghigo E, Ho K, Melmed S (2010) A consensus on criteria for cure of acromegaly. J Clin Endocrinol Metab 95:3141–3148
Katznelson L, Atkinson JL, Cook DM, Ezzat SZ, Hamrahian AH, Miller KK (2011) American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of acromegaly–2011 update. Endocr Pract 17(suppl 4):1–44
Giustina A, Chanson P, Kleinberg D, Bronstein MD, Clemmons DR, Klibanski A, van der Lely AJ, Strasburger CJ, Lamberts SW, Ho KK, Casanueva FF, Melmed S (2014) Expert consensus document: a consensus on the medical treatment of acromegaly. Nat Rev Endocrinol 10:243–248
Growth Hormone Research Society, Pituitary Society (2004) Biochemical assessment and long-term monitoring in patients with acromegaly: statement from a joint consensus conference of the Growth Hormone Research Society and the Pituitary Society. J Clin Endocrinol Metab 89:3099–3102
Woodmansee WW, Carmichael J, Kelly D, Katznelson L (2015) American Association of Clinical Endocrinologists and American College of Endocrinology disease state clinical review: postoperative management following pituitary surgery. Endocr Pract 21:832–838
Melmed S, Kleinberg DL, Bonert V, Fleseriu M (2014) Acromegaly: assessing the disorder and navigating therapeutic options for treatment. Endocr Pract 20(Suppl 1):7–17
Varadhan L, Reulen RC, Brown M, Clayton RN (2016) The role of cumulative growth hormone exposure in determining mortality and morbidity in acromegaly: a single centre study. Pituitary 19:251–261
Abreu A, Tovar AP, Castellanos R, Valenzuela A, Giraldo CM, Pinedo AC, Guerrero DP, Barrera CA, Franco HI, Ribeiro-Oliveira A Jr, Vilar L, Jallad RS, Duarte FG, Gadelha M, Boguszewski CL, Abucham J, Naves LA, Musolino NR, de Faria ME, Rossato C, Bronstein MD (2016) Challenges in the diagnosis and management of acromegaly: a focus on comorbidities. Pituitary 19:448–457
Caron P, Bex M, Cullen DR, Feldt-Rasmussen U, Pico Alfonso AM, Pynka S, Racz K, Schopohl J, Tabarin A, Valimaki MJ (2004) Group for Lanreotide Autogel Long-Term Study on Acromegaly: one-year follow-up of patients with acromegaly treated with fixed or titrated doses of lanreotide autogel. Clin Endocrinol 60:734–740
Chanson P, Borson-Chazot F, Kuhn JM, Blumberg J, Maisonobe P, Delemer B (2008) Lanreotide Acromegaly Study Group: control of IGF-I levels with titrated dosing of lanreotide autogel over 48 weeks in patients with acromegaly. Clin Endocrinol 69:299–305
Kyriakakis N, Chau V, Lynch J, Orme SM, Murray RD (2014) Lanreotide autogel in acromegaly—a decade on. Expert Opin Pharmacother 15:2681–2692
Murray RD, Melmed S (2008) A critical analysis of clinically available somatostatin analog formulations for therapy of acromegaly. J Clin Endocrinol Metab 93:2957–2968
Tutuncu Y, Berker D, Isik S, Ozuguz U, Akbaba G, Kucukler FK, Aydin Y, Guler S (2012) Comparison of octreotide LAR and lanreotide autogel as post-operative medical treatment in acromegaly. Pituitary 15:398–404
Melmed S, Cook D, Schopohl J, Goth MI, Lam KS, Marek J (2010) Rapid and sustained reduction of serum growth hormone and insulin-like growth factor-1 in patients with acromegaly receiving lanreotide autogel therapy: a randomized, placebo-controlled, multicenter study with a 52 week open extension. Pituitary 13:18–28
Caron PJ, Bevan JS, Petersenn S, Flanagan D, Tabarin A, Prevost G, Maisonobe P, Clermont A (2014) Tumor shrinkage with lanreotide autogel 120 mg as primary therapy in acromegaly: results of a prospective multicenter clinical trial. J Clin Endocrinol Metab 99:1282–1290
Neggers SJ, Pronin V, Balcere I, Lee MK, Rozhinskaya L, Bronstein MD, Gadelha MR, Maisonobe P, Sert C, van der Lely AJ, LEAD Study Group (2015) Lanreotide autogel 120 mg at extended dosing intervals in patients with acromegaly biochemically controlled with octreotide LAR: the LEAD study. Eur J Endocrinol 173:313–323
Annamalai AK, Webb A, Kandasamy N, Elkhawad M, Moir S, Khan F, Maki-Petaja K, Gayton EL, Strey CH, O’Toole S, Ariyaratnam S, Halsall DJ, Chaudhry AN, Berman L, Scoffings DJ, Antoun NM, Dutka DP, Wilkinson IB, Shneerson JM, Pickard JD, Simpson HL, Gurnell M (2013) A comprehensive study of clinical, biochemical, radiological, vascular, cardiac, and sleep parameters in an unselected cohort of patients with acromegaly undergoing presurgical somatostatin receptor ligand therapy. J Clin Endocrinol Metab 98:1040–1050
Sagvand BT, Khairi S, Haghshenas A, Swearingen B, Tritos NA, Miller KK, Klibanski A, Nachtigall LB (2016) Monotherapy with lanreotide depot for acromegaly: long-term clinical experience in a pituitary center. Pituitary 19:437–447
Salvatori R, Nachtigall LB, Cook DM, Bonert V, Molitch ME, Blethen S, Chang S, The SALSA Study Group (2010) Effectiveness of self- or partner-administration of an extended-release aqueous-gel formulation of lanreotide in lanreotide-naive patients with acromegaly. Pituitary 13:115–122
Bevan JS, Newell-Price J, Wass JA, Atkin SL, Bouloux PM, Chapman J, Davis JR, Howlett TA, Randeva HS, Stewart PM, Viswanath A (2008) Home administration of lanreotide autogel® by patients with acromegaly, or their partners, is safe and effective. Clin Endocrinol 68:343–349
Salvatori R, Woodmansee WW, Molitch M, Gordon MB, Lomax KG (2014) Lanreotide extended-release aqueous-gel formulation, injected by patient, partner or healthcare provider in patients with acromegaly in the United States: 1-year data from the SODA registry. Pituitary 17:13–21
Carmichael JD (2012) Lanreotide depot deep subcutaneous injection: a new method of delivery and its associated benefits. Patient Prefer Adherence 6:73–82
Colao A, Bronstein MD, Freda P, Gu F, Shen CC, Gadelha M, Fleseriu M, van der Lely AJ, Farrall AJ, Hermosillo Resendiz K, Ruffin M, Chen Y, Sheppard M, Pasireotide C2305 Study Group (2014) Pasireotide versus octreotide in acromegaly: a head-to-head superiority study. J Clin Endocrinol Metab 99:791–799
Gadelha MR, Bronstein MD, Brue T, Coculescu M, Fleseriu M, Guitelman M, Pronin V, Raverot G, Shimon I, Lievre KK, Fleck J, Aout M, Pedroncelli AM, Colao A (2014) Pasireotide C2402 Study group: pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trial. Lancet Diab Endocrinol 2:875–884
Travers K, Sallum RH, Burns MD, Barr CE, Beattie MS, Pashos CL, Luce BR (2015) Characteristics and temporal trends in patient registries: focus on the life sciences industry, 1981–2012. Pharmacoepidemiol Drug Saf 24:389–398
Kauppinen-Makelin R, Sane T, Reunanen A, Valimaki MJ, Niskanen L, Markkanen H, Loyttyniemi E, Ebeling T, Jaatinen P, Laine H, Nuutila P, Salmela P, Salmi J, Stenman UH, Viikari J, Voutilainen E (2005) A nationwide survey of mortality in acromegaly. J Clin Endocrinol Metab 90:4081–4086
Howlett TA, Willis D, Walker G, Wass JA, Trainer PJ, UK Acromegaly Register Study Group (UKAR-3) (2013) Control of growth hormone and IGF1 in patients with acromegaly in the UK: responses to medical treatment with somatostatin analogues and dopamine agonists. Clin Endocrinol 79:689–699
Cuevas-Ramos D, Carmichael JD, Cooper O, Bonert VS, Gertych A, Mamelak AN, Melmed S (2015) A structural and functional acromegaly classification. J Clin Endocrinol Metab 100:122–131
Sesmilo G, Gaztambide S, Venegas E, Pico A, Del PC, Blanco C, Torres E, Alvarez-Escola C, Fajardo C, Garcia R, Camara R, Bernabeu I, Soto A, Villabona C, Serraclara A, Halperin I, Alcazar V, Palomera E, Webb SM, REA Investigators (2013) Changes in acromegaly treatment over four decades in Spain: analysis of the Spanish Acromegaly Registry (REA). Pituitary 16:115–121
Agustsson TT, Baldvinsdottir T, Jonasson JG, Olafsdottir E, Steinthorsdottir V, Sigurdsson G, Thorsson AV, Carroll PV, Korbonits M, Benediktsson R (2015) The epidemiology of pituitary adenomas in Iceland, 1955–2012: a nationwide population-based study. Eur J Endocrinol 173:655–664
Portocarrero-Ortiz LA, Vergara-Lopez A, Vidrio-Velazquez M, Uribe-Diaz AM, Garcia-Dominguez A, Reza-Albarran AA, Cuevas-Ramos D, Melgar V, Talavera J, Rivera-Hernandez AJ, Valencia-Mendez CV, Mercado M, Mexican Acromegaly Registry Group (2016) The Mexican acromegaly registry: clinical and biochemical characteristics at diagnosis and therapeutic outcomes. J Clin Endocrinol Metab 101:3997–4004
Petersenn S, Buchfelder M, Gerbert B, Franz H, Quabbe HJ, Schulte HM, Grussendorf M, Reincke M (2009) Age and sex as predictors of biochemical activity in acromegaly: analysis of 1485 patients from the German Acromegaly Register. Clin Endocrinol 71:400–405
Bex M, Abs R, T’Sjoen G, Mockel J, Velkeniers B, Muermans K, Maiter D (2007) AcroBel–the Belgian registry on acromegaly: a survey of the ‘real-life’ outcome in 418 acromegalic subjects. Eur J Endocrinol 157:399–409
Fieffe S, Morange I, Petrossians P, Chanson P, Rohmer V, Cortet C, Borson-Chazot F, Brue T, Delemer B, French Acromegaly Registry (2011) Diabetes in acromegaly, prevalence, risk factors, and evolution: data from the French Acromegaly Registry. Eur J Endocrinol 164:877–884
Mestron A, Webb SM, Astorga R, Benito P, Catala M, Gaztambide S, Gomez JM, Halperin I, Lucas-Morante T, Moreno B, Obiols G, de Pablos P, Paramo C, Pico A, Torres E, Varela C, Vazquez JA, Zamora J, Albareda M, Gilabert M (2004) Epidemiology, clinical characteristics, outcome, morbidity and mortality in acromegaly based on the Spanish Acromegaly Registry (Registro Espanol de Acromegalia, REA). Eur J Endocrinol 151:439–446
Schofl C, Franz H, Grussendorf M, Honegger J, Jaursch-Hancke C, Mayr B, Schopohl J, Participants of the German Acromegaly Register (2013) Long-term outcome in patients with acromegaly: analysis of 1344 patients from the German Acromegaly Register. Eur J Endocrinol 168:39–47
Sherlock M, Reulen RC, Aragon-Alonso A, Ayuk J, Clayton RN, Sheppard MC, Hawkins MM, Bates AS, Stewart PM (2014) A paradigm shift in the monitoring of patients with acromegaly: last available growth hormone may overestimate risk. J Clin Endocrinol Metab 99:478–485
Colao A, Ferone D, Marzullo P, Lombardi G (2004) Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev 25:102–152
Ben-Shlomo A, Sheppard MC, Stephens JM, Pulgar S, Melmed S (2011) Clinical, quality of life, and economic value of acromegaly disease control. Pituitary 14:284–294
Attanasio AF, Mo D, Erfurth EM, Tan M, Ho KY, Kleinberg D, Zimmermann AG, Chanson P (2010) Prevalence of metabolic syndrome in adult hypopituitary growth hormone (GH)-deficient patients before and after GH replacement. J Clin Endocrinol Metab 95:74–81
Pokrajac A, Frystyk J, Flyvbjerg A, Trainer PJ (2009) Pituitary-independent effect of octreotide on IGF1 generation. Eur J Endocrinol 160:543–548
Gleeson HK, Lissett CA, Shalet SM (2005) Insulin-like growth factor-I response to a single bolus of growth hormone is increased in obesity. J Clin Endocrinol Metab 90:1061–1067
Nam SY, Lee EJ, Kim KR, Lee HC, Nam MS, Cho JH, Huh KB (1996) Long-term administration of acipimox potentiates growth hormone response to growth hormone-releasing hormone by decreasing serum free fatty acid in obesity. Metabolism 45:594–597
Ayuk J, Clayton RN, Holder G, Sheppard MC, Stewart PM, Bates AS (2004) Growth hormone and pituitary radiotherapy, but not serum insulin-like growth factor-I concentrations, predict excess mortality in patients with acromegaly. J Clin Endocrinol Metab 89:1613–1617
Dekkers OM, Biermasz NR, Pereira AM, Romijn JA, Vandenbroucke JP (2008) Mortality in acromegaly: a metaanalysis. J Clin Endocrinol Metab 93:61–67
Alexopoulou O, Bex M, Abs R, T’Sjoen G, Velkeniers B, Maiter D (2008) Divergence between growth hormone and insulin-like growth factor-1 concentrations in the follow-up of acromegaly. J Clin Endocrinol Metab 93:1324–1330
Arosio M, Reimondo G, Malchiodi E, Berchialla P, Borraccino A, De Marinis L, Pivonello R, Grottoli S, Losa M, Cannavo S, Minuto F, Montini M, Bondanelli M, De Menis E, Martini C, Angeletti G, Velardo A, Peri A, Faustini-Fustini M, Tita P, Pigliaru F, Borretta G, Scaroni C, Bazzoni N, Bianchi A, Appetecchia M, Cavagnini F, Lombardi G, Ghigo E, Beck-Peccoz P, Colao A, Terzolo, M (2012) Predictors of morbidity and mortality in acromegaly: an Italian survey. Eur J Endocrinol 167:189–198
Colao A, Bronstein MD, Freda P, Gu F, Shen CC, Gadelha M, Fleseriu M, van der Lely AJ, Farrall AJ, Hermosillo RK, Ruffin M, Chen Y, Sheppard M (2014) Pasireotide versus octreotide in acromegaly: a head-to-head superiority study. J Clin Endocrinol Metab 99:791–799
Reid TJ, Jin Z, Shen W, Reyes-Vidal CM, Fernandez JC, Bruce JN, Kostadinov J, Post KD, Freda PU (2015) IGF-1 levels across the spectrum of normal to elevated in acromegaly: relationship to insulin sensitivity, markers of cardiovascular risk and body composition. Pituitary 18:808–819
Biermasz NR, Dekker FW, Pereira AM, van Thiel SW, Schutte PJ, van Dulken H, Romijn JA, Roelfsema F (2004) Determinants of survival in treated acromegaly in a single center: predictive value of serial insulin-like growth factor I measurements. J Clin Endocrinol Metab 89:2789–2796
Freda PU (2009) Monitoring of acromegaly: what should be performed when GH and IGF-1 levels are discrepant? Clin Endocrinol 71:166–170
Niculescu D, Purice M, Coculescu M (2013) Insulin-like growth factor-I correlates more closely than growth hormone with insulin resistance and glucose intolerance in patients with acromegaly. Pituitary 16:168–174
Parkinson C, Ryder WD, Trainer PJ (2001) The relationship between serum GH and serum IGF-I in acromegaly is gender-specific. J Clin Endocrinol Metab 86:5240–5244
Leung KC, Doyle N, Ballesteros M, Waters MJ, Ho KK (2000) Insulin regulation of human hepatic growth hormone receptors: divergent effects on biosynthesis and surface translocation. J Clin Endocrinol Metab 85:4712–4720
Ciresi A, Amato MC, Pivonello R, Nazzari E, Grasso LF, Minuto F, Ferone D, Colao A, Giordano C (2013) The metabolic profile in active acromegaly is gender-specific. J Clin Endocrinol Metab 98:E51–E59
Suliman M, Jenkins R, Ross R, Powell T, Battersby R, Cullen DR (1999) Long-term treatment of acromegaly with the somatostatin analogue SR-lanreotide. J Endocrinol Invest 22:409–418
Colao A, Pivonello R, Cappabianca P, Briganti F, Tortora F, Auriemma RS, De Martino MC, Marzullo P, Lombardi G (2005) Effect of gender and gonadal status on the long-term response to somatostatin analogue treatment in acromegaly. Clin Endocrinol 63:342–349
Cook DM, Ludlam WH, Cook MB (1999) Route of estrogen administration helps to determine growth hormone (GH) replacement dose in GH-deficient adults. J Clin Endocrinol Metab 84:3956–3960
Lombardi G, Minuto F, Tamburrano G, Ambrosio MR, Arnaldi G, Arosio M, Chiarini V, Cozzi R, Grottoli S, Mantero F, Bogazzi F, Terzolo M, Tita P, Boscani PF, Colao A (2009) Efficacy of the new long-acting formulation of lanreotide (lanreotide autogel) in somatostatin analogue-naive patients with acromegaly. J Endocrinol Invest 32:202–209
Schopohl J, Strasburger CJ, Caird D, Badenhoop K, Beuschlein F, Droste M, Plockinger U, Petersenn S (2011) Efficacy and acceptability of lanreotide autogel® 120 mg at different dose intervals in patients with acromegaly previously treated with octreotide LAR. Exp Clin Endocrinol Diabetes 119:156–162
Orlewska E, Kos-Kudla B, Sowinski J, Sworczak K, Zgliczynski W (2015) Dosage and costs of lanreotide autogel 120 mg administered as part of routine acromegaly care in Poland—two years of data from Lanro-Study. Endokrynol Pol 66:142–148
Kasuki L, Marques NV, Nuez MJ, Leal VL, Chinen RN, Gadelha MR (2013) Acromegalic patients lost to follow-up: a pilot study. Pituitary 16:245–250
Delemer B, Chanson P, Foubert L, Borson-Chazot F, Chabre O, Tabarin A, Weryha G, Cortet-Rudelli C, Raingeard I, Reznik Y, Reines C, Bisot-Locard S, Castinetti F (2014) Patients lost to follow-up in acromegaly: results of the ACROSPECT study. Eur J Endocrinol 170:791–797
Strasburger CJ, Karavitaki N, Stormann S, Trainer PJ, Kreitschmann-Andermahr I, Droste M, Korbonits M, Feldmann B, Zopf K, Sanderson VF, Schwicker D, Gelbaum D, Haviv A, Bidlingmaier M, Biermasz NR (2016) Patient-reported outcomes of parenteral somatostatin analogue injections in 195 patients with acromegaly. Eur J Endocrinol 174:355–362
Mazziotti G, Floriani I, Bonadonna S, Torri V, Chanson P, Giustina A (2009) Effects of somatostatin analogs on glucose homeostasis: a metaanalysis of acromegaly studies. J Clin Endocrinol Metab 94:1500–1508
Caron PJ, Petersenn S, Houchard A, Sert C, Bevan JS, PRIMARYS Study Group (2017) Glucose and lipid levels with lanreotide autogel 120 mg in treatment-naive patients with acromegaly: data from the PRIMARYS study. Clin Endocrinol 86:541–551
Carmichael JD, Bonert VS, Nuno M, Ly D, Melmed S (2014) Acromegaly clinical trial methodology impact on reported biochemical efficacy rates of somatostatin receptor ligand treatments: a meta-analysis. J Clin Endocrinol Metab 99:1825–1833
Acknowledgements
The authors wish to thank the patients and investigators of each participating center in this study. The authors also thank Olga V. Gambetti, formerly of Ipsen Biopharmaceuticals, Inc., for her assistance with this study and Kathleen Allen, study manager. Sarah Mizne, PharmD and Lynanne McGuire, PhD, of MedVal Scientific Information Services, LLC, provided professional writing and editorial assistance which was funded by Ipsen Biopharmaceuticals, Inc. This manuscript was prepared according to the International Society for Medical Publication Professionals’ “Good Publication Practice for Communicating Company-Sponsored Medical Research: the GPP3 Guidelines” and the International Committee of Medical Journal Editors’ “Uniform Requirements for Manuscripts Submitted to Biomedical Journals.”
Funding
This study was sponsored by Ipsen Biopharmaceuticals, Inc., Basking Ridge, NJ.
Author information
Authors and Affiliations
Contributions
All authors contributed equally and each was involved in study design, data acquisition, or data analysis/ interpretation and in drafting or critically revising the manuscript. All authors reviewed the final manuscript and gave approval for submission.
Corresponding author
Ethics declarations
Conflict of interest
Roberto Salvatori: Consultant/Advisory Board: Pfizer, Ionis Pharmaceutical, Novo Nordisk, Novartis; Research support: Ipsen, Novartis, Pfizer, Novo Nordisk, Chiasma, Millendo, Strongbridge, Prolor. Murray B. Gordon: Research support: Ipsen, Chiasma, Novartis, Novo Nordisk, Opko, Pfizer, Strongbridge, Teva. Whitney W. Woodmansee: Clinical trial investigator: Ipsen, Novo Nordisk, Versartis, Pfizer; Consultant/Advisory Board: Corcept, Genentech, Ipsen. Adriana G. Ioachimescu: Research support: Novartis, Ipsen, Chiasma, Pfizer; Consultant/Advisory Board: Chiasma, Ionis, and Ipsen. Beloo Mirakhur and David Cox: Full-time employees: Ipsen Biopharmaceuticals, Inc. Don W. Carver: Ipsen consultant. Mark E. Molitch: Research support: Ipsen, Novartis, Bayer, Prolor, Novo Nordisk, Johnson and Johnson; Consultant: Corcept, Ipsen, Novartis, Novo Nordisk, Merck, Pfizer.
Informed consent
All eligible patients signed a statement of informed consent, and the day on which the informed consent form was signed was considered the enrollment date.
Research involving human participants
The SODA study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice Guidelines, current Food and Drug Administration regulations and guidelines, local ethical and legal requirements, and the United States Code of Federal Regulations and the Health Insurance Portability and Accountability Act for the collection, transmission, and storage of study data. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statement on the welfare of animals
This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Salvatori, R., Gordon, M.B., Woodmansee, W.W. et al. A multicenter, observational study of lanreotide depot/autogel (LAN) in patients with acromegaly in the United States: 2-year experience from the SODA registry. Pituitary 20, 605–618 (2017). https://doi.org/10.1007/s11102-017-0821-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-017-0821-y