Abstract
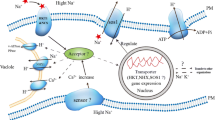
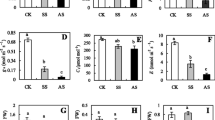
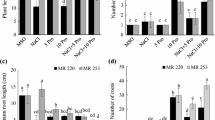
Alkali stress is an important agricultural problem that affects plant metabolism, specifically root physiology. In this study, using two rice cultivars differing in alkali resistance, we investigated the physiological and molecular responses of rice plants to alkali stress. Compared to the alkali-sensitive cultivar (SC), the alkali-tolerant cultivar (TC) maintained higher photosynthesis and root system activity under alkali stress. Correspondingly, the Na+ content in its shoots was much lower, and the contents of mineral ions (e.g., K+, NO3 −, and H2PO4 −) in its roots was higher than those of the SC. These data showed that the metabolic regulation of roots might play a central role in rice alkali tolerance. Gene expression differences between the cultivars were much greater in roots than in shoots. In roots, 46.5% (20 of 43) of selected genes indicated over fivefold expression differences between cultivars under alkali stress. The TC had higher root system activity that might protect shoots from Na+ injury and maintain normal metabolic processes. During adaptation of TC to alkali stress, OsSOS1 (salt overly sensitive protein 1) may mediate Na+ exclusion from shoots or roots. Under alkali stress, SC could accumulate Na+ up to toxic concentrations due to relatively low expression of OsSOS1 in shoots. It possibly harmed chloroplasts and influenced photorespiration processes, thus reducing NH4 + production from photorespiration. Under alkali stress, TC was able to maintain normal nitrogen metabolism, which might be important for resisting alkali stress.
Similar content being viewed by others
Abbreviations
- AKT:
-
low affinity K+ transporter
- AS:
-
asparagine synthetase
- AST:
-
alkali stress treatment
- GDH:
-
glutamate dehydrogenase
- GOGAT:
-
glutamate synthase
- GS:
-
glutamine synthetase
- HAK:
-
KUP/HAK/KT K+ transporter
- HKT:
-
high affinity K+ transporter
- NHX:
-
Na+/H+ exchanger
- NiR:
-
nitrite reductase
- NR:
-
nitrate reductase
- OA:
-
organic acid
- P5CS:
-
δ1-pyrroline-5-carboxylate synthetase
- ProDH:
-
proline dehydrogenase
- SC:
-
alkali-sensitive cultivar
- SOS:
-
salt overly sensitive
- SST:
-
salt stress treatment
- TC:
-
alkali-tolerant cultivar
References
Chen W., Cui P., Sun H. et al.: Comparative effects of salt and alkali stresses on organic acid accumulation and ionic balance of seabuckthorn (Hippophae rhamnoides L.). — Ind. Crop Prod. 30: 351–358, 2009.
Comas L.H., Eissenstat D.M., Lakso A.N.: Assessing root death and root system dynamics in a study of grape canopy pruning. — New Phytol. 147: 171–178, 2000.
Crawford N.M., Glass A.D.M.: Molecular and physiological aspects of nitrate uptake in plants. — Trends Plant Sci. 3: 389–395, 1998.
Gao C., Wang Y., Liu G. et al.: Expression profiling of salinityalkali stress responses by large-scale expressed sequence tag analysis in Tamarix hispid. — Plant Mol. Biol. 66: 245–258, 2008.
Horie T., Hauser F., Schroeder J.I.: HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. — Trends Plant Sci. 14: 660–668, 2009.
Jain M., Nijhawan A., Tyagi A.K., Khurana J.P.: Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. — Biochem. Biophys. Res. Commun. 345: 646–651, 2006.
Kant S., Bi Y.M., Rothstein S.J.: Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. — J. Exp. Bot. 62: 1499–1509, 2010.
Kawanabe S., Zhu T.: Degeneration and conservational trial of Aneurolepidium chinense grassland in Northern China. — J. Japan. Grassl. Sci. 39: 91–99, 1991.
Kusano M., Tabuchi M., Fukushima A. et al.: Metabolomics data reveal a crucial role of cytosolic glutamine synthetase 1;1 in coordinating metabolic balance in rice. — Plant J. 66: 456–466, 2011.
Läuchli A., Lüttge U.: Salinity in the soil environment. — In: Tanji K.K. (ed.): Salinity: Environment-Plants-Molecules. Pp. 21–23. Kluwer Academic Publ., Boston 2002.
Livak K.J., Schmittgen T.D.: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta (CT)) Method. — Methods 25: 402–408, 2001.
Lutts S., Kinet J., Bouharmont J.: NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. — Ann Bot. 78: 389–398, 1996.
Martinez-Atienza J., Jiang X., Garciadeblas B. et al.: Conservation of the salt overly sensitive pathway in rice. — Plant Physiol. 143: 1001–1012, 2007.
Munns R., Tester M.: Mechanisms of salinity tolerance. — Annu. Rev. Plant Biol. 59: 651–681, 2008.
Quinet M., Ndayiragije A., Lefèvre I. et al.: Putrescine differently influences the effect of salt stress on polyamine metabolism and ethylene synthesis in rice cultivars differing in salt resistance. — J. Exp. Bot. 61: 2719–2733, 2010.
Shi D., Sheng Y.: Effect of various salt-alkaline mixed stress conditions on sunflower seedlings and analysis of their stress factors. — Environ. Exp. Bot. 54: 8–21, 2005.
Shi D., Wang D.: Effects of various salt-alkaline mixed stresses on Aneurolepidium chinense (Trin.) Kitag. — Plant Soil 271: 15–26, 2005.
Shi D., Yin L.: Difference between salt (NaCl) and alkaline (Na2CO3) stresses on Pucinellia tenuiflora (Griseb.) Scribn. et Merr. plants. — Acta Bot. Sin. 35: 144–149, 1993.
Shi H., Quintero F.J., Pardo J.M., Zhu J.K.: The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. — Plant Cell 14: 465–477, 2002.
Touraine B., Grignon N., Grignon C.: Charge balance in NO3--fed soybean: Estimation of K+ and carboxylate recirculation. — Plant Physiol. 88: 605–612, 1998.
Wang H., Han J., Wu Z. et al.: Alteration of nitrogen metabolism in rice variety ‘Nipponbare’ induced by alkali stress. — Plant Soil 355: 131–147, 2012.
Wang H., Wu Z., Chen Y. et al.: Effects of salt and alkali stresses on growth and ion balance in rice (Oryza sativa L.). — Plant Soil Environ. 57: 286–294, 2011.
Wang X.L.: Carboxylic acid. — In: Wang X.L. (ed.): Organic Chemistry. Pp. 149–150. Higher Education Press, Beijing 2001.
Yang C., Chong J., Li C. et al.: Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. — Plant Soil 294: 263–276, 2007.
Yang C., Xu H., Wang L. et al.: Comparative effects of salt-stress and alkali-stress on the growth, photosynthesis, solute accumulation, and ion balance of barley plants. — Photosynthetica 47: 79–86, 2009.
Yang F., Liang Z., Wang Z.: [Effect of soda saline-sodic stress on the panicle traits and yield components of rice variety Changbai 9.] — North. China Agron. J. 25: 59–61, 2010. In Chinese]
Zang A., Xu X., Neill S., Cai W.: Overexpression of OsRAN2 in rice and Arabidopsis renders transgenic plants hypersensitive to salinity and osmotic stress. — J. Exp. Bot. 61: 777–789, 2010.
Zhang Z.: Laboratory Manual of Plant Physiology. Higher Education Press, Beijing 2004.
Zhu J.K.: Regulation of ion homeostasis under salt stress. — Curr. Opin. Plant Biol. 6: 441–445, 2003.
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgements: This study was supported by the National Natural Science Foundation of China Project (31300192), Project of the Jilin Provincial Government (No. 20106023), and Basic Research Project by Jilin Provincial Government (No. 20090567). We thank International Science Editing (ISE) for language editing.
Rights and permissions
About this article
Cite this article
Wang, H., Lin, X., Cao, S. et al. Alkali tolerance in rice (Oryza sativa L.): growth, photosynthesis, nitrogen metabolism, and ion homeostasis. Photosynthetica 53, 55–65 (2015). https://doi.org/10.1007/s11099-015-0079-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11099-015-0079-4