Abstract
Background
Chronic non-cancer pain may affect up to 51% of the general population. Pharmacist interventions have shown promise in enhancing patient safety and outcomes. However, our understanding of the scope of pharmacists’ interventions remains incomplete.
Aim
Our goal was to characterise pharmacists’ interventions for the management of chronic non-cancer pain.
Method
Medline, Embase, PsycINFO via Ovid, CINAHL via EBSCO databases and the Cochrane Library were systematically searched. Abstracts and full texts were independently screened by two reviewers. Data were extracted by one reviewer, and validated by the second. Outcomes of studies were charted using the dimensions of the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT).
Results
Forty-eight reports were included. Interventions ensuring appropriate drug prescription occurred in 37 (79%) studies. Patient education and healthcare professional education were reported in 28 (60%) and 5 (11%) studies, respectively. Therapy monitoring occurred in 17 (36%) studies. Interventions regularly involved interprofessional collaboration. A median of 75% of reported outcome domains improved due to pharmacist interventions, especially patient disposition (adherence), medication safety and satisfaction with therapy.
Conclusion
Pharmacists’ interventions enhanced the management of chronic non-cancer pain. Underreported outcome domains and interventions, such as medication management, merit further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impact statements
-
Pharmacists are well-positioned to improve the management of chronic non-cancer pain.
-
Pharmacists should consider multimodal, interprofessional interventions for the management of chronic non-cancer pain.
-
Future opportunities for pharmacists lie in holistic, patient-centred approaches such as medication management.
Introduction
Chronic non-cancer pain (CNCP) affects a substantial portion of the population, with at least one-fifth of individuals in the US and Europe experiencing this condition at one point during their lives (19–51%) [1, 2]. CNCP induces a significant burden on overall quality of life, often including depression and insomnia [3,4,5,6]. CNCP is more common among women [7], older adults [8] and people from poor socioeconomic background [9]. As defined by the International Association for the Study of Pain, chronic pain, including CNCP, lasts for over three months [10]. There are various causes of CNCP, often necessitating effective multimodal management strategies, combining physical, psychological and pharmacological therapies [11].
Pharmacists play crucial roles in enhancing medication safety in a variety of settings and patient populations, offering services such as prescription validation, patient counselling and therapeutic drug monitoring [12]. Their involvement in medication reviews (MRs) and dosage adjustments contribute to appropriate CNCP management [13]. Indeed, positive effects have been found by three preceding systematic reviews regarding pharmacists’ interventions in CNCP management: a 2011 systematic review and meta-analysis found that patient education by pharmacists reduced pain intensity [14]; a 2014 systematic review exploring pharmacists performing MRs also found lower levels of pain [15]; and a more recent systematic review and meta-analysis reported similar effects for interventions not limited to education or MRs [16]. Altogether, current evidence suggests that pharmacists enhance the quality of care provided to patients with CNCP.
However, these three reviews only provided limited insight into the type and structure of these interventions [14,15,16]. When pharmacists seek to contribute to CNCP care, they often lack a comprehensive understanding of which interventions they could implement in this context. This scoping review is intended to bridge this information gap.
Aim
The aim of this scoping review was to concisely yet comprehensively identify, describe and categorise the current range and attributes of pharmacists’ interventions and services for managing patients with CNCP. The secondary objective was to descriptively synthesise reported outcomes.
Method
Protocol and design
Prior to starting this review, a protocol was published [17], and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses’ extension for scoping reviews (PRISMA-Scr) [18] was followed in reporting.
Eligibility criteria
The studies included involved adult patients (≥ 18 years) suffering from CNCP. Author-defined CNCP or pain described as lasting more than three months were both accepted. Patients with cancer-related pain and patients addicted to opioids were excluded. All pharmacist interventions aimed at patients with CNCP were included, except for interventions solely targeting opioid addiction or opioid tapering. If the intervention included opioid tapering but also focused on improving CNCP care (i.e. by providing patient education), the study was included. Interventions delivered in any healthcare setting were accepted, as were trials with or without controls. Primary literature with any study design was included (except case studies). Non-peer-reviewed papers, conference abstracts, conference proceedings, editorials, commentaries and literature reviews were excluded. There were no restrictions on dates or the language of publication.
Information sources
Sources of evidence were retrieved from Medline, Embase and PsycINFO databases via Ovid, as well as from the CINAHL database via EBSCO and the Cochrane Library. Additionally, backward citation chasing was performed on the studies retrieved using the Citationchaser tool [19]. The first 100 citations, sorted by relevance, were screened using Google Scholar.
Search
Ovid was used to create the search string for the Medline database, and the search string was validated using articles from previous systematic reviews [14,15,16] that met the inclusion criteria as seed papers and in collaboration with the University of Basel’s Medical Library. The search strategy was translated for other databases using the Systematic Review Accelerator® [20]. The search consisted of two thematic search blocks, ‘pharmacists’ and ‘chronic non-cancer pain’. Each block combined MeSH or Emtree terms as well as free text searches limited to title and abstract. The search strings are shown in Supplementary Information 1. The search was conducted on 12 October 2023.
Selecting sources of evidence
The selection processes were carried out using Covidence® systematic review software (Veritas Health Innovation, Melbourne, Australia.). Duplicate articles were also removed with Covidence®. Titles and abstracts were independently screened by both authors based on the inclusion and exclusion criteria. The full texts of the studies retained were retrieved. The full-text information was independently compared with the inclusion and exclusion criteria by both authors, and a final decision was made. In cases of disagreement, the authors again resolved them through discussion.
Data items
Pre-defined data items were retrieved as per the review protocol [17]. General study details and methodological information were tabulated. Information about the pharmacist interventions (types and characteristics) was extracted. Aims, outcomes, results and conclusions were also included.
Data charting
Data were charted using the three dimensions of intervention type, setting and outcomes. Intervention types were charted using an adapted framework based on the definition of clinical pharmacy published in a European Society of Clinical Pharmacy policy paper [21] (Table 1). It is worth noting that, each intervention could combine multiple intervention types. Studies were also charted according to their healthcare setting, distinguishing between acute care (e.g. hospitals), ambulatory care (e.g. outpatient clinics in hospitals) and primary care (e.g. primary care centres, community pharmacies). Furthermore, various outcomes reported by the study authors were charted following an adapted methodology used by Gondora et al. [22] and using the core outcome domains for chronic pain clinical trials in the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials’ (IMMPACT) guidelines [23]. The IMMPACT domains encompass: (1) pain intensity, (2) physical function, (3) emotional well-being, (4) participant ratings of satisfaction or improvement with therapy, (5) symptoms and ADEs, and (6) participant disposition (e.g. adherence to the therapy). An evaluation of costs was added as a seventh domain. The number of domains each study covered and whether they improved, stayed the same or deteriorated was assessed. When a domain improved, one point was awarded; when it deteriorated, one point was subtracted; and when no change occurred in the domain, no points were awarded. A half point was added or subtracted for partial improvements or partial deteriorations. A domain was considered to have partially improved or deteriorated if not all reported variables for that domain showed improvement or deterioration. Each study’s ratio of points awarded per outcome domain was calculated. The median improvement in each domain and the total number of times each domain was mentioned were reported. These two numbers made it possible to compute the median percentage of improved domains. To depict the distribution of medians, their inter-quartile range (IQR) was reported. In addition, the reported outcome domains were compared for different types of intervention. Using those categories, data charting was completed by the first reviewer (AG) and verified by the second (CMM). In cases of disagreement, the reviewers sought consensus through discussion.
Synthesis of results
The results were analysed and described in a narrative synthesis, and the results were categorised to assess the frequency of intervention types.
Results
Selection of sources of evidence
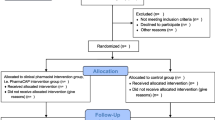
The combined search found 3758 published articles across the five databases. Based on our defined criteria, 48 reports were included [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71]. One study report [59] was a secondary analysis of another [32], so those papers’ insights were combined. Among these 47 different interventional studies, 41 were identified through the systematic search and 7 through citation chasing and hand searching. Figure 1 depicts the study’s PRISMA flow diagram [18].
PRISMA-Scr flow diagram [18]. Created with BioRender.com
Characteristics of sources of evidence
The studies included had varied characteristics, with 29 (62%) originating from North America, 13 (38%) from Europe, three (6%) from Australia, and two (4%) from Asia. Sixteen (34%) studies were pre–post studies, 11 (23%) were cross-sectional studies, six (13%) were randomised controlled trials, four (9%) were chart reviews and 10 (21%) employed other study designs.
Patients with any form of CNCP were included in the majority of studies (n = 42, 90%), although some interventions targeted specific subpopulations. Two (4%) interventions were directed at patients with arthritis, two (4%) targeted patients with migraine, and one (2%) was aimed specifically at patients with neuropathic pain.
Results of individual sources of evidence
The information and charted interventions extracted from each article included are presented in Table 2.
Synthesis of results
The included studies described interventions in different settings. Ambulatory care settings were reported on in 21(45%) studies, primary care settings in 18 (38%) and acute care settings in 6 (13%), with 1 (2%) in a university (2%) and 1 (2%) in a prison service facility.
Intervention characteristics varied across the included studies. Multi-component interventions were present in 30 (64%) studies, and single-component interventions were presented in 17 (36%). Drug prescription interventions were presented in 37 (79%) studies, of which 20 (43%) reported face-to-face MRs, 14 (30%) reported remote MRs, 11 (23%) reported medication management and 5 (11%) reported medication reconciliation. Educational interventions were reported in 31 (66%) studies, with 5 (11%) describing educational interventions involving healthcare professionals (HPs) and 28 (60%) describing them with patients. Monitoring interventions were described in 17 (36%) studies, and compounding was reported in one (2%).
Pharmacists frequently cooperated with other HPs when providing care for patients with CNCP. Physicians were involved in 42 (89%) studies, nurses in 14 (30%) and physiotherapists in 6 (13%). Other less frequently involved professions included psychologists (n = 4, 9%), dieticians (n = 2, 4%), social workers (n = 2, 4%), occupational therapists (n = 2, 4%), behavioural therapists (n = 1, 2%) and biologists (n = 1, 2%).
The scope of pharmacists’ practice differed across the reported interventions. In 9 (19%) studies, pharmacists possessed prescribing rights and managed their patients independently; in the 38 (81%) remaining studies, pharmacists either recommended medication therapy optimisations to physicians and/or advised patients directly.
The studies reported a median of two outcome domains (interquartile range (IQR): 1–4) and, of these, a median of 1.5 domains improved (IQR: 1–2), corresponding to a median of 75% improved outcomes per study. Forty studies reported on symptoms and ADEs, 24 on pain intensity, 22 on physical function, 18 on emotional well-being, 14 on satisfaction with therapy, 9 on patient disposition and 6 on the costs of the intervention. Except for physical function, all the domains had positive outcomes in the majority of studies. The highest number of positive outcomes were reported in the domain of adherence (8/9 studies were positive, 89%), followed by symptoms and ADEs (34/40 positive, 85%), satisfaction with therapy (11.5/14 positive, 82%), the costs of the intervention (4/6 positive, 67%), pain intensity (15/24 positive, 63%), emotional well-being (10/18 positive, 56%) and physical function (9/22 positive, 41%). More information on the respective intervention types is shown in Table 3 and Supplementary Information 2.
Discussion
Statement of key findings
This scoping review included 47 studies and identified a diverse range of pharmacist interventions aimed at improving the care of patients with CNCP. These interventions took place in a variety of healthcare settings, with most occurring in ambulatory care, followed by primary and then acute care. The most frequently reported interventions focused on drug prescriptions. Among these, face-to-face MRs were the most common, followed by remote MRs, medication management and medication reconciliation. Studies often combined multiple intervention types. Out of seven possible outcome domains—pain intensity, physical functioning, psychological well-being, patient satisfaction, symptoms and ADEs, patient disposition, and costs—study authors reported a median of two domains, of which a median of 1.5 domains of intervention showed positive outcomes.
Strengths and weaknesses
This review has some limitations. Although our search strategy comprised some diseases closely associated with chronic pain (e.g. migraine), it was not exhaustive in terms of individual conditions that may be associated with chronic pain. Therefore, some studies reporting pharmacist interventions targeting other specific diseases (e.g. arthritis), but not specifically mentioning chronic pain (or a synonym thereof), may have been missed. Full-text data were extracted by one reviewer alone, and a second reviewer verified the retrieved information, so this may have introduced bias. With few studies originating from Asia, and none from Africa or South America, this may limit the generalisability of the results of this review.
The main strength of this scoping review is the extensive search over five large databases, including a citation chase and an extensive hand search. Another strength is the presentation of reported outcomes on the IMMPACT domains, which represent the relevant aspects of CNCP care.
Interpretation
In this scoping review, a median of 75% of reported outcome domains improved thanks to pharmacist interventions, aligning with three existing systematic reviews that consistently reported the positive effects of pharmacist interventions for CNCP management [14,15,16]. Bennet et al. reported on pain intensity, symptoms and ADEs, emotional well-being and satisfaction with therapy [14]; Hadi et al. reported on pain intensity, physical function and satisfaction with treatment [15]; and Thapa et al. reported on pain intensity, physical function, emotional well-being, satisfaction with therapy and costs [16]. To the best of our knowledge, there have been no systematic reviews covering all of the outcome domains that should be considered according to the IMMPACT guidelines [23]. Improvements in pain intensity and satisfaction with therapy outcomes were reported by all three reviews, aligning with this review’s qualitative synthesis of author-reported outcomes.
The outcome domains of symptoms and ADE (85%) and of patient disposition (89%) in the included studies had high percentages of improved outcomes. Bennet et al. found a significant reduction of ADEs in the interventions reviewed [14]. However, none of the existing systematic reviews considered adherence. Pharmacists are often seen as medication experts, and there is evidence in studies on other diseases that pharmacists can enhance adherence [72]. This suggests that the evidence of the benefits of pharmacist interventions on symptoms and ADEs and on patient disposition, reported in this scoping review, are plausible.
The predominance and greater impact of interventions including some form of MR or patient education found in this review align with previous research in this area. Existing systematic reviews have explored the impact of pharmacist interventions in CNCP management, with some focusing specifically on patient education [14] or MRs [15], indicating the importance of these intervention types. A third systematic review, that did not restrict intervention types, identified 8 out of 14 studies as MR interventions [16], which explains why MR has been implemented repeatedly. Interestingly, in this scoping review, remote and face-to-face MR showed the same median improvement. However, remote MR interventions reported fewer outcome domains than face-to-face MR. This emphasises the potentially more comprehensive approach of a face-to-face MR that allows for patient involvement. The highest median improvements in outcomes were shown by interventions including medication reconciliation, HP education and medication management. However, these three intervention types only comprised a few reports each, making generalisation more difficult. Medication management, in particular, is promising because it involves the pharmacist holistically in CNCP care. It also requires continuous follow-up and patient contact, both of which are known to contribute to improved patient outcomes [11, 73].
In the included studies, pharmacists mostly took a multimodal approach by combining diverse interventions (e.g. face-to-face MR and patient education) and collaborated with other professionals, mainly physicians and nurses, to deliver their interventions, which is widely recommended [11, 73]. This recommendation is supported by this scoping review’s finding that multi-component interventions reported more improved outcomes than single-component interventions.
Further research
Further research into pharmacist interventions in CNCP care should prioritise less-researched interventions, such as medication management, alongside investigating less conventional roles for pharmacists, such as independent prescribing. Emphasis should be placed on direct patient contact, adequate follow-up and a multimodal approach. In addition, to facilitate the development of rational and effective interventions, more research is needed to determine which types of pharmacist interventions have the highest impact on reported outcomes. Comprehensive reporting should include all the relevant IMMPACT domains, particularly the effect of pharmacist interventions on patient disposition (adherence), patient satisfaction and costs.
Conclusion
This scoping review revealed a diverse landscape of pharmacist interventions targeting patients with CNCP. Intervention types that addressed appropriate drug prescribing, educated healthcare professionals or patients, monitored treatments and offered compounding were identified. Pharmacist activities often combined multiple intervention types via interprofessional teams. A median of 75% of outcomes in all the reported outcome domains improved after the implementation of pharmacist interventions. However, the effects of pharmacist interventions on important outcome domains, such as costs, patient disposition and satisfaction with the therapy, and their role in interventions such as medication management, remain underreported and require further study.
References
Yong RJ, Mullins PM, Bhattacharyya N. Prevalence of chronic pain among adults in the United States. Pain. 2022;163(2):e328–32.
Reid KJ, Harker J, Bala MM, et al. Epidemiology of chronic non-cancer pain in Europe: narrative review of prevalence, pain treatments and pain impact. Curr Med Res Opin. 2011;27(2):449–62.
Hadi MA, McHugh GA, Closs SJ. Impact of chronic pain on patients’ quality of life: a comparative mixed-methods study. J Patient Exp. 2019;6(2):133–41.
Jank R, Gallee A, Boeckle M, Fiegl S, Pieh C. Chronic pain and sleep disorders in primary care. Pain Res Treat. 2017;2017:9081802. https://doi.org/10.1155/2017/9081802
Currie SR, Wang J. More data on major depression as an antecedent risk factor for first onset of chronic back pain. Psychol Med. 2005;35(9):1275–82.
Banks SM, Kerns RD. Explaining high rates of depression in chronic pain: A diathesis-stress framework. Psychol Bull. 1996;119(1):95.
Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. 2004;8(5):397–411.
Helme RD, Gibson SJ. The epidemiology of pain in elderly people. Clin Geriatr Med. 2001;17(3):417–31.
Janevic MR, McLaughlin SJ, Heapy AA, et al. Racial and socioeconomic disparities in disabling chronic pain: findings from the health and retirement study. J Pain. 2017;18(12):1459–67.
Treede R-D, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain. 2019;160(1):19–27.
Schwan J, Sclafani J, Tawfik VL. Chronic pain management in the elderly. Anesthesiol Clin. 2019;37(3):547–60.
Barber N, Willson A. Churchill's clinical pharmacy survival guide. Churchill Livingstone; 1999.
Dispennette R, Hall LA, Elliott DP. Activities of palliative care and pain management clinical pharmacists. Am J Health Syst Pharm. 2015;72(12):999–1000.
Bennett MI, Bagnall A-M, Raine G, et al. Educational interventions by pharmacists to patients with chronic pain: systematic review and meta-analysis. Clin J Pain. 2011;27(7):623–30.
Hadi MA, Alldred DP, Briggs M, et al. Effectiveness of pharmacist-led medication review in chronic pain management: systematic review and meta-analysis. Clin J Pain. 2014;30(11):1006–14.
Thapa P, Lee SWH, Kc B, et al. Pharmacist-led intervention on chronic pain management: a systematic review and meta-analysis. Br J Clin Pharmacol. 2021;87(8):3028–42.
Goetschi AN, Meyer-Massetti C. Pharmacist Interventions for Non-cancer Pain: A Protocol for a Scoping Review. Zenodo. 2023. https://doi.org/10.5281/zenodo.7762443
McGowan J, Straus S, Moher D, et al. Reporting scoping reviews—PRISMA ScR extension. J Clin Epidemiol. 2020;123:177–9.
Haddaway NR, Grainger MJ, Gray CT. Citationchaser: A tool for transparent and efficient forward and backward citation chasing in systematic searching. Res Synth Methods. 2022;13(4):533–45.
Clark JM, Sanders S, Carter M, et al. Improving the translation of search strategies using the Polyglot Search Translator: a randomized controlled trial. J Med Libr Assoc. 2020;108(2):195–207.
Dreischulte T, van den Bemt B, Steurbaut S, et al. European Society of Clinical Pharmacy definition of the term clinical pharmacy and its relationship to pharmaceutical care: a position paper. Int J Clin Pharm. 2022;44(4):837–42.
Gondora N, Versteeg SG, Carter C, et al. The role of pharmacists in opioid stewardship: A scoping review. Res Social Adm Pharm. 2022;18(5):2714–47.
Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106(3):337–45.
Alibaud R, De Saint-Denis AM, De Saint-Denis DM, et al. Conciliation médicamenteuse et non médicamenteuse avec processus du parcours patient en consultation douleur chronique. Douleurs. 2016;17(4):205–10.
Barrachina J, Margarit C, Andreu B, et al. Therapeutic alliance impact on analgesic outcomes in a real-world clinical setting: An observational study. Acta Pharm. 2022;72(4):529–45.
Bauters TG, Devulder J, Robays H. Clinical pharmacy in a multidisciplinar team for chronic pain in adults. Acta Clin Belg. 2008;63(4):247–50.
Bellnier TJ, Brown GW, Ortega T et al. Description of collaborative, fee-for-service, office-based, pharmacist-directed medical cannabis therapy management service for patients with chronic pain. J Am Pharm Assoc (2003). 2022;62(3):889–96.
Bhimji H, Landry E, Jorgenson D. Impact of pharmacist-led medication assessments on opioid utilization. Can Pharm J (Ott). 2020;153(3):148–52.
Boren LL, Locke AM, Friedman AS, et al. Team-based medicine: Incorporating a clinical pharmacist into pain and opioid practice management. Pm & R. 2019;11(11):1170–7.
Briggs M, Closs SJ, Marczewski K, et al. A feasibility study of a combined nurse/pharmacist-led chronic pain clinic in primary care. Qual Prim Care. 2008;16(2):91–4.
Bright D, Saadeh C, DeVuyst-Miller S, et al. Pharmacist consult reports to support pharmacogenomics report interpretation. Pharmgenomics Pers Med. 2020;13:719–24.
Bruhn H, Bond CM, Elliott AM et al. Pharmacist-led management of chronic pain in primary care: results from a randomised controlled exploratory trial. BMJ Open. 2013;3(4).
Chelminski PR, Ives TJ, Felix KM, et al. A primary care, multi-disciplinary disease management program for opioid-treated patients with chronic non-cancer pain and a high burden of psychiatric comorbidity. BMC Health Serv Res. 2005;5(1):3.
Chen M, Patel T, Chang F. The impact of a primary care, pharmacist-driven intervention in patients with chronic non-cancer pain-a pilot study. Pharmacy (Basel). 2020;8(3):08.
Coffey CP, Ulbrich TR, Baughman K et al. The effect of an interprofessional pain service on nonmalignant pain control. Am J Health Syst Pharm. 2019;76(Supplement_2):S49–S54.
Conley MP, Elmes AT, Roberts JR, et al. Developing an interprofessional team to support patients prescribed long-term high-dose opioid therapy. J Interprof Care. 2023;37(2):320–4.
Cox N, Tak CR, Cochella SE, et al. Impact of pharmacist previsit input to providers on chronic opioid prescribing safety. J Am Board Fam Med. 2018;31(1):105–12.
Dawson KG, Mok V, Wong JGM, Bhalla A. Deprescribing initiative of NSAIDs (DIN): Pharmacist-led interventions for pain management in a federal correctional setting. Can Pharm J (Ott). 2023;156(2):85–93. https://doi.org/10.1177/17151635221149712
DeBar L, Mayhew M, Benes L, et al. A primary care-based cognitive behavioral therapy intervention for long-term opioid users with chronic pain: a randomized pragmatic trial. Ann Intern Med. 2022;175(1):46–55.
Dole EJ, Murawski MM, Adolphe AB, et al. Provision of pain management by a pharmacist with prescribing authority. Am J Health Syst Pharm. 2007;64(1):85–9.
Duvivier H, Houck M, Ressler E, et al. A multidisciplinary chronic pain management clinic in an indian health service facility. Fed Pract. 2015;32(8):24–30.
Faley B, Brooks A, Vartan CM, et al. Impact of clinical pharmacist practitioner-driven high opioid dose reevaluation in veterans with chronic non-cancer pain. J Pain Palliat Care Pharmacother. 2022;36(4):249–59.
Fong GR. The role of the pharmacist in an operant conditioning program for chronic pain patients. Drug Intell Clin Pharm. 1975;9(2):68–75.
Gammaitoni AR, Gallagher RM, Welz M, et al. Palliative pharmaceutical care: a randomized, prospective study of telephone-based prescription and medication counseling services for treating chronic pain. Pain Med. 2000;1(4):317–31.
Hadi MA, Alldred DP, Briggs M, et al. Effectiveness of a community based nurse-pharmacist managed pain clinic: A mixed-methods study. Int J Nurs Stud. 2016;53:219–27.
Hay EM, Foster NE, Thomas E, et al. Effectiveness of community physiotherapy and enhanced pharmacy review for knee pain in people aged over 55 presenting to primary care: pragmatic randomised trial. BMJ. 2006;333(7576):995.
Hoffmann W, Herzog B, Mühlig S, et al. Pharmaceutical care for migraine and headache patients: a community-based randomized intervention. Ann Pharmacother. 2008;42(12):1804–13.
Jorgenson D. Evaluation of a pharmacist-led interprofessional chronic pain clinic in Canada. Can Pharm J (Ott). 2023;156(5):265–71.
Joypaul S, Kelly F, King MA. Turning pain into gain: evaluation of a multidisciplinary chronic pain management program in primary care. Pain Med. 2018;20(5):925–33.
Keen A, McCrate B, McLennon S, et al. Influencing nursing knowledge and attitudes to positively affect care of patients with persistent pain in the hospital setting. Pain Manag Nurs. 2017;18(3):137–43.
Kientz JE, Fitzsimmons DS, Schneider PJ. Reducing medication use in a chronic pain management program. Am J Hosp Pharm. 1983;40(12):2156–8.
Kroner BA, Billups SJ, Garrison KM, et al. Actual versus projected cost avoidance for clinical pharmacy specialist-initiated medication conversions in a primary care setting in an integrated health system. J Manag Care Pharm. 2008;14(2):155–63.
Lagisetty P, Smith A, Antoku D, et al. A physician-pharmacist collaborative care model to prevent opioid misuse. Am J Health Syst Pharm. 2020;77(10):771–80.
Maleki S, Szmerling J, Thiruvasagan M, Seah G, Gu G. Pharmacist ambulatory pain services for a chronic non-cancer pain clinic: a descriptive study. J Pharm Pract Res. 2023;53:241–47. https://doi.org/10.1002/jppr.1875
Manzur V, Mirzaian E, Huynh T et al. Implementation and assessment of a pilot, community pharmacy-based, opioid pain medication management program. J Am Pharm Assoc (2003). 2020;60(3):497–502.
Mayfield K, Nguyen H, Smith C, et al. The impact and value of a pharmacist within a persistent pain management service. J Pharm Pract Res. 2021;51(6):505–10.
McDermott ME, Smith BH, Elliott AM, et al. The use of medication for chronic pain in primary care, and the potential for intervention by a practice-based pharmacist. Fam Pract. 2006;23(1):46–52.
Minguez Marti A, Cerda Olmedo G, Valia Vera JC, et al. Effectiveness of a pharmaceutical care unit for the control of severe chronic pain. Farm Hosp. 2005;29(1):37–42.
Neilson AR, Bruhn H, Bond CM, et al. Pharmacist-led management of chronic pain in primary care: costs and benefits in a pilot randomised controlled trial. BMJ Open. 2015;5(4): e006874.
Norman JL, Kroehl ME, Lam HM, et al. Implementation of a pharmacist-managed clinic for patients with chronic nonmalignant pain. Am J Health Syst Pharm. 2017;74(16):1229–35.
Petkova VB. Education for arthritis patients: a community pharmacy based pilot project. Pharm Pract (Granada). 2009;7(2):88–93.
Read RW, Krska J. Targeted medication review: Patients in the community with chronic pain. Int J Pharm Pract. 1998;6(4):216–22.
Richet E, Ferret L, Gaboriau L, et al. Use of dronabinol in the treatment of resistant neuropathic pain: Feedback from patients followed in a multidisciplinary pain center. Ann Pharm Fr. 2022;10:10.
Rife T, Zhao M, Im J, et al. Evaluating implementation of a consult to reduce new combination opioid and benzodiazepine prescriptions at the San Francisco Veterans Affairs Health Care System. J Am Coll Clin Pharm. 2021;4(7):808–18.
Semerjian M, Durham MJ, Mirzaian E, et al. Clinical pharmacy services in a multidisciplinary specialty pain clinic. Pain Pract. 2019;19(3):303–9.
Skomo ML. Impact of pharmacist interventions on seeking of medical care by migraineurs. J Am Pharm Assoc (2003). 2008;48(1):32–7.
Slipp M, Burnham R. Medication management of chronic pain: A comparison of 2 care delivery models. Can Pharm J (Ott). 2017;150(2):112–7.
Takahashi N, Kasahara S, Yabuki S. Development and implementation of an inpatient multidisciplinary pain management program for patients with intractable chronic musculoskeletal pain in Japan: preliminary report. J Pain Res. 2018:201–11.
Tilli T, Hunchuck J, Dewhurst N, et al. Opioid stewardship: implementing a proactive, pharmacist-led intervention for patients coprescribed opioids and benzodiazepines at an urban academic primary care centre. BMJ Open Qual. 2020;9(2): e000635.
Uejima K, Hayasaka M, Kato J, et al. Hospital-pharmacy cooperative training and drug-taking compliance in outpatients with chronic pain: a case-control study. Integr Pharm Res Pract. 2019;8:63–74.
Weidman-Evans E, Jacobs TF, Isherwood P, et al. Impact of a pharmacistdeveloped protocol on the cardiac monitoring of methadone in chronic noncancer pain management. J Am Pharm Assoc. 2003;2009:e102–9.
Rubio-Valera M, Serrano-Blanco A, Magdalena-Belío J, et al. Effectiveness of pharmacist care in the improvement of adherence to antidepressants: a systematic review and meta-analysis. Ann Pharmacother. 2011;45(1):39–48.
National Institute for Health and Care Excellence. Chronic pain (primary and secondary) in over 16s: Assessment of all chronic pain and management of chronic primary pain. (2021, April 7). [NICE guideline No. 192. https://www.nice.org.uk/guidance/ng193. 2021. Accessed 14.12.2023.
Acknowledgements
We wish to acknowledge the support received from Dr Hannah Ewald, University Medical Library, University of Basel, Basel, Switzerland.
Funding
No specific funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Goetschi, A.N., Meyer-Massetti, C. Characterising pharmacists’ interventions in chronic non-cancer pain care: a scoping review. Int J Clin Pharm (2024). https://doi.org/10.1007/s11096-024-01741-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11096-024-01741-x