Abstract
Background
Adverse drug events contribute to rising health care costs. Clinical pharmacists can reduce their risks by identifying and solving drug-related problems (DRPs) through medication review.
Aim
To develop an economic model to determine whether medication reviews performed by clinical pharmacists could lead to a reduction in health care costs associated with the prevention of potential adverse drug events.
Method
Two pharmacists performed medication reviews during ward rounds in an internal medicine setting over one year. Avoided costs were estimated by monetizing five categories of DRPs (improper drug selection, drug interactions, untreated indications, inadequate dosages, and drug use without an indication). An expert panel assessed potential adverse drug events and their probabilities of occurrence for 20 randomly selected DRPs in each category. The costs of adverse drug events were extracted from internal hospital financial data. A partial economic study from a hospital perspective then estimated the annual costs avoided by resolving DRPs identified by 3 part-time clinical pharmacists (0.9 full-time equivalent) from 2019 to 2020. The return on investment (ROI) of medication review was calculated.
Results
The estimated annual avoided costs associated with the potential adverse drug events induced by 676 DRPs detected was € 304,170. The cost of a 0.9 full-time equivalent clinical pharmacist was € 112,408. Extrapolated to 1 full-time equivalent, the annual net savings was € 213,069 or an ROI of 1–1.71. Sensitivity analyses showed that the economic model was robust.
Conclusion
This economic model revealed the positive financial impact and favorable return on investment of a medication review intervention performed by clinical pharmacists. These findings should encourage the future deployment of a pharmacist-led adverse drug events prevention program.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impact statements
-
This study highlighted the importance of employing an economic model that assesses costs comprehensively using real hospital costs for more accurate results and revealing the return on investment (ROI) of clinical pharmacy activities.
-
The calculation model created is robust, innovative, easy to use and applicable in other healthcare context.
-
This project confirmed that medication reviews performed by a clinical pharmacist in internal medicine wards prevent adverse drug event-related expenses and is cost-effective.
-
Pharmacists should focus their interventions on patients at a high risk of adverse drug events or on drugs that can cause very expensive adverse drug events to ensure even greater cost-effectiveness.
Introduction
Adverse drug events (ADEs) resulting from medication errors (MEs) have a significant negative impact on patient safety. However, they also contribute to rising health care costs, which have reached an estimated USD 177 billion in the United States, € 370 million in Sweden, and more than € 1 billion in Germany [1,2,3]. ADEs cause avoidable health care consumption through unplanned consultations with general practitioners or emergency departments, hospitalizations, and prolonged hospital stays. One study stated that 4 emergency department visits per 1000 inhabitants were related to ADEs [4]. Hospitalization rates due to ADEs vary from 3.25 to 25% [5,6,7,8], and the prevalence of in-hospital ADEs ranges from 3.2 to 5.6% depending on the country [6].
Clinical pharmacists can reduce the risks of ADEs by preventing medication errors (MEs) through optimizing drug therapy and improving patient medication adherence, thus indirectly reducing unnecessary expenditures [7,8,9,10,11,12,13,14]. Clinical pharmacists can also save money by negotiating drug purchase prices with suppliers, ensuring proper inventory management, and promoting the safe, efficient, appropriate, and economical use of pharmaceuticals within hospitals.
Most research on the economic dimensions of clinical pharmacists’ activities has been conducted in the United States. Systematic reviews have shown the positive economic impacts of hospital and community pharmacists’ activities [15,16,17,18,19]. Studies have also assessed the cost savings association with using less expensive drugs, promoting generic or biosimilar drugs, substituting parenteral drugs for oral forms, optimizing treatment duration, and enhancing medication management [20,21,22,23,24,25,26,27,28,29]. Additionally, studies measured cost avoidance through pharmacist-led interventions, such as patient-tailored activities to improve adherence or optimize drug therapy on wards [9, 20,21,22,23, 30,31,32,33,34,35,36,37,38,39,40,41,42].
Pharmacoeconomic models, such as cost minimization, cost-effectiveness, cost-benefit, or cost-utility analyses, are not easy to grasp and apply for pharmacists. They are not always clearly described in published studies and there is often significant between-study variability in healthcare settings, methodologies, and results. An absence of standardized economic evaluation methods in this field and the lack of comprehensive economic studies complicates data interpretation, limits reproducibility, and constrains the applicability of their results to different healthcare systems. Overcoming the challenge of reducing healthcare costs and demonstrating a return on investment (ROI) remains a major obstacle to expanding the employment of clinical pharmacists in many countries.
Aim
This study aimed to develop a simple, easily applicable economic model based on literature and adapted to Switzerland’s healthcare system to demonstrate that clinical pharmacists’ medication review (MR) activities could reduce hospital expenditures by preventing costly ADEs among inpatients.
Ethics approval
It was not necessary to undergo an ethical compliance evaluation procedure for a pharmacoeconomic study.
Method
Pharmaceutical intervention
This study defined pharmacist-led intervention as identifying drug-related problems (DRPs) through inpatient medication reviews and providing treatment optimization recommendations during ward rounds. According to the Pharmaceutical Care Network Europe (PCNE) [43] and Hepler and Strand definition [44], a DRP is an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes. We considered that unsolved DRPs may have led to acute ADEs during hospitalization. ADEs and related costs occurring after discharge were not included in the study. The ADE definition of Bates et al. was employed in this research [45].
Design, setting, and study population
To develop the economic model, two clinical pharmacists collected data on avoided costs related to different categories of DRPs over a 12-month period (January–December 2019) in a 200-bed internal medicine division of a Swiss university hospital. Five categories were chosen because of their frequent occurrence in a previous study conducted in the same department: improper drug selection, drug interactions, untreated indications, inadequate dosages, and drug use without an indication [46]. Only DRPs for which treatment optimization recommendations were implemented by physicians were considered in the analysis.
Economic model development
Study perspective
The partial economic study evaluated the avoided costs associated with ADE prevention from a health care provider’s perspective. In this study, the hospital, which is publicly funded, was considered a payer and provider.
Selection of DRPs and identification of potential ADEs
Among the 538 DRPs detected by the two pharmacists in one year, 20 in each of the five categories of DRPs, each associated with a different clinical condition, were randomly selected. An expert panel (one attending physician in internal medicine and one senior clinical pharmacist) assessed the potential acute ADEs that could have occurred with the highest probability without the intervention of the clinical pharmacist for each of these 100 DRPs. They evaluated these 100 DRPs individually using their clinical experience, knowledge, and the medical literature. They estimated the probability of an ADE occurring for each DRP according to the stratification probability scale described by Nesbit et al. [21]. This scale assigns a probability of 0, 0.01, 0.1, 0.4, and 0.6 to occurrences (corresponding to no probability, very low probability, low probability, medium probability, and high probability, respectively). The most likely related potential ADE was matched to the probability of occurrence of each clinical DRP by consensus. In the absence of consensus, an attending physician specializing in clinical pharmacology could be asked to make the final decision. Examples of DRPs are presented in electronic supplementary material 1.
Avoided costs of the potential ADEs related to DRPs
The hospital’s cost accounting team calculated the avoided costs by preventing potential ADEs using real data on the direct hospital costs that would have been incurred due to additional medical care and/or extended length of stay. They use the standardized REKOLE® method [47], which establishes how each hospital stay consumes direct health resources, called work units (e.g., drugs consumed, minutes in the operating room, minutes of care), and indirect resources leading to expenses, called unit costs of load centres (which are groupings of expenses necessary to implement an activity, such as infrastructure, sterilization, anaesthesia, imaging, or cleaning). This microcosting method makes it possible to estimate the cost of a hospital stay as well as the additional costs of an extended stay or readmission.
To obtain an accurate representative cost for each ADE identified by the expert panel, it was considered translatable into a disease and was associated with a corresponding International Classification of Diseases version 10 (ICD-10) [48, 49] classification code. The accounting team extracted data on every hospital stay for each code of interest during 2017, and they analyzed and adjusted their costs to obtain a realistic median cost for the hospital associated with managing those conditions. Costs were calculated in Swiss francs.
Avoided costs by DRP category
A median cost was applied to each DRP’s potential ADE and weighted by that ADE’s probability of occurrence, as assigned by the expert panel. These were used to calculate the avoided cost for each of the 100 DRPs. Table 1 illustrates the calculation process using the example of an identified ADE. Once the avoided costs were known for each of the 100 DRPs, a mean and median avoided cost by DRP category could be calculated. The economic model used median costs because of the cost data’s non-Gaussian distribution. Costs were expressed in Swiss francs and were converted into Euros (€) using 2017’s mean exchange rate (€ 1 = CHF 1.11156946).
Return on investment analysis
Investment costs
Investment costs were the hospital’s spending on medication review activities, mainly annual expenditure on clinical pharmacists’ salaries in 2019–2020. The part-time activities of the three pharmacists corresponded to a 0.9 FTE position.
Avoided costs
Avoided costs were the estimated hospital costs saved based on all the DRPs collected that led to prescription modifications over a one-year period (April 2019–March 2020) by three part-time clinical pharmacists. The previously estimated median avoided costs were applied to each clinical intervention implemented, according to their DRP categories, and cumulated.
Return-on-investment calculation
The clinical pharmacists’ economic impact was assessed by measuring the ROI of their medication review activities. The hospital’s annual investment in pharmacists’ salaries was subtracted from the annual net savings generated by ADE prevention. This resulted in a net saving with which to calculate an ROI ratio to the annual investment.
Sensitivity analyses
Three sensitivity analyses were carried out to assess the robustness of the economic analysis, to identify the study’s limitations and to understand how to best interpret the economic evaluation. The first analysis decreased the cost of any ADEs by 2.5 times (60%), the second analysis decreased their probability of occurrence by half, and the third analysis weighted 1.5 times (33.33%) decrease in costs of any ADE and fixed the probability of occurrence to 0.05.
Results
Economic model development
Avoided costs of the potential ADEs related to DRPs
The expert panel’s evaluation of 100 DRPs identified 33 different potentially preventable ADEs. Several DRPs led to the same ADE. These ADEs and the institutional costs calculated for their management are presented in electronic supplementary material 2. Median ADE costs ranged from € 591 to € 17,384, demonstrating that some of the DRPs detected could prevent ADEs that proved very costly to the hospital. Serious ADEs affecting a patient’s quality of life or causing severe complications were the most expensive in terms of hospital management (e.g., femoral neck fracture, osteomyelitis, intracerebral haemorrhage). The suggested probabilities of occurrence varied from 0 to 0.4, with none being assigned a probability of 0.6. The expert panel assessed that 29 of the 100 clinical situations would not cause immediate ADEs and were assigned a probability of occurrence of 0; 8 cases were assigned a probability of 0.01; 55 were assigned a probability of 0.1; and 8 cases were assigned a probability of 0.4.
Avoided costs by DRP category
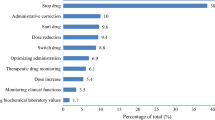
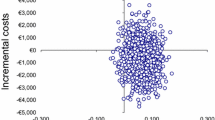
Median costs associated with each category of DRP are presented in Table 2. They ranged from € 0 to € 897. The distribution of individual costs by DRP category is shown in Fig. 1.
Return-on-investment analysis
Over 12 months, the three part-time pharmacists performed 144 medication reviews for 973 patients (6–7 patients per review), detecting and solving 676 DRPs. Of these, 184 involved inadequate doses (over- or underdosing), 178 involved untreated indications, 152 involved drug interactions, 108 involved drug use without an indication, and 54 involved improper drug selection. The total avoided cost of these 676 prevented DRPs, based on the median avoided costs by category, was estimated at € 304,170. Most of this was saved by identifying untreated indications (€ 127,146). Detecting DRPs involving drug use without an indication did not save the hospital money because most were associated with drugs that did not induce acute ADEs that might prolong hospitalization. The total avoided costs by DRP category are shown in Table 3.
The financial investment required to enable the three part-time clinical pharmacists (totalling a 0.9 FTE position) to carry out their medication review activities was € 112,408. The annual net savings were € 191,762. Extrapolating this result to 1 FTE clinical pharmacist’s position came to € 213,069, giving an ROI of 1–1.71.
Sensitivity analyses
The total annual avoided costs, net savings, and ROI were calculated using three sensitivity analyses, as presented in Table 4. The economic impact remained positive in the sensitivity analyses where costs were reduced by 60% and where the probability of occurrence was made very low. The third sensitivity analysis illustrated the economic model’s break-even point.
Discussion
This study demonstrated that medication reviews performed by clinical pharmacists during ward rounds could significantly reduce hospital costs by preventing ADEs. Our results showed a cost-saving ratio or ROI of €1.71 for each €1 invested in clinical pharmacy activities. Our calculated benefits fall within the range of findings from prior studies.
Few studies evaluated the ROI of clinical pharmacist activities in a hospital setting, and even fewer quantified the avoided costs associated with ADE prevention or calculated a median avoided cost for each DRP category. To the best of our knowledge, this study is the first to use real institutional costs from analytical accounting through a microcosting approach.
Major pharmacoeconomic reviews on clinical pharmacy interventions showed a wide range of ROIs from 1 to 75.84 [15,16,17,18], reflecting the variability in study types included, interventions evaluated and costs considered. For example, the research perspective adopted can impact the costs considered.
In Schumock and Perez’s review, the median ROIs ranged from 1 to 4.81 [16, 17], corroborating our own findings. Studies that monetized ADEs using costs per DRP category calculated an ROI ranging from 1 to 14 [8, 9, 21, 22, 50, 51]. All these studies used a slightly different DRP cost calculation method (e.g., fixed cost for any ADE; fixed ADE occurrence probability; costs saved directly when a drug was stopped or changed.).
Two European studies [22, 51] used the microcosting method developed by Rottenkobler et al. [3] to assign an avoided cost to a prevented ADE associated with a probability of occurrence score, as per Nesbit et al. [21]. The ROIs measured were 1–8.64 and 1–1.76. They fixed the value of a prevented ADE to be € 1,057 and € 1,079, respectively. Rottenkobler’s cost calculation method was quite similar to ours in that it incorporated the sum of the single cost components associated with inpatient treatments for ADEs based on cost centres. This way of quantifying ADEs enables more accurate and transparent cost assessments.
The differences between prior studies illustrated the variabilities in health care systems, settings, types of costs, cost calculations, or definitions of a pharmaceutical intervention or an ADE. This makes data comparisons, data extrapolations and generalizations challenging.
One strength of our study is the method of calculating avoided costs, which was closer to the reality on wards than what other researchers have used to date. We put values on ADEs using real costs derived from our hospital’s in-house accounting data rather than estimating costs from the literature. This was innovative because we calculated a median avoided cost for five DRP categories by costing real clinical situations at risk of ADEs and prolonged hospital stays. The actual hospital cost of managing each of these situations could be calculated by translating ADE into a disease or condition whose cost was easily quantifiable. The use of ICD-10 to translate an ADE is novel and practical as long as it is compatible with the health care cost valuation system of a country, as is the case in Switzerland.
Costs were saved in each category of DRP detected by the pharmacists, except drug use without an indication. Potential ADEs from this DRP category were calculated to have a median cost of € 0 because in more than 50% of the 20 cases quantified, the potential ADEs would only occur in the long term (e.g., osteoporosis, clostridium difficile or respiratory infections caused by proton pump inhibitors) without additional costs during hospitalization. These pharmaceutical interventions remain relevant and could be valuable to studies examining ROI calculations from a societal perspective. It is worth noting that DRP detection also aims to impact clinical and human factors. Although identifying a DRP may not only save money, it can also improve the patient’s quality of life.
Sensitivity analyses showed that our economic model was robust. The break-even point demonstrated that intervening solely on low-probability or low-cost ADEs was worth the effort financially.
Our study suggests that pharmacists need to prioritize their interventions on risky DRPs to avoid drug-related morbidity costs. They should focus their interventions primarily on patients who pose a high clinical and financial risk to the hospital, regardless of whether they are common or uncommon. (e.g., fragile populations, poly-morbidity/medication).
Pharmacists should also focus on drugs with a high risk of acute ADEs or medication errors (e.g., drugs with narrow therapeutic range). To achieve this, they should seek the support of digital tools to help identify these situations. New existing systems can flag patients at risk of ADEs by using different triggers in electronic patient records. Moreover, they are cost-effective [52,53,54,55,56].
Unlike other studies [8, 9, 21, 31, 39, 57], ours did not include direct costs saved in medication (e.g., an inpatient’s prescribed expensive compound to a less expensive one, switching from an intravenous to an oral form, or using a biosimilar instead of an original drug). If other types of costs, such as humanistic, indirect, and intangible costs, were considered in this analysis, the savings would have been even increased.
Despite our innovative cost calculation method, the present study had some limitations. The data sample for measuring costs associated with different categories of DRPs was limited. A larger sample size would have resulted in more accurate, realistic costs. Random data selection from such a small sample may not be sufficiently representative of the actual distribution of identified risk situations. The expert panel may also have influenced the costs used since other evaluators might have chosen differently at this stage of the project. The precise attribution of avoided costs to a DRP is highly variable because it depends on the DRP’s actual clinical impact, which is difficult to predict. For example, we calculated the savings from detecting a drug interaction DRP to be € 725. Other studies estimated these avoided costs to be between € 285 and € 14,943 [31, 33, 34, 38]. The way in which costs are quantified can greatly influence this value. With this model, each DRP was linked to a single potential ADE. Some clinical situations, might involve multiple ADEs stemming from one DRP, potentially increasing the avoided cost. This overlapping impact was not assessed in this analysis. The precise definition of pharmacists’ clinical interventions and categories of DRP may also influence cost calculations and could explain interstudy cost variations. There are no standardized definitions of these interventions. Finally, only the pharmacists' direct salary costs were used for cost investments calculations. Including indirect costs would lower the net benefit and ROI.
Conducting a comparative cost‒benefit study is recommended to assess the best financial impact of clinical pharmacists. It will enable an estimation of the actual differences in hospitalization costs between inpatients with DRPs undergoing or not undergoing medication review. Previous research teams have already conducted cost‒benefit analyses and cost-minimization studies to evaluate the cost-effectiveness of pharmacists' interventions [29, 52, 58,59,60]. Many cost-effectiveness publications have utilized cost-utility studies to measure benefits in quality-adjusted life years [35, 59, 61,62,63,64,65,66,67,68]. Alternatively, using prospective randomized controlled studies or before-and-after economic studies could enhance the measurement of avoided costs, as demonstrated by some research groups [57, 58, 67, 69,70,71]
Conclusion
The present study showed the positive economic impact and favourable cost–benefit ratio of a medication review intervention performed by clinical pharmacists during internal medicine ward rounds. An innovative calculation model used real hospital costs to estimate the savings that a pharmacy department could deliver to a hospital with a pharmacist-led ADE prevention program—this could be a cost justification method in the pharmacy department’s budgeting.
It thus seems sensible for a hospital to invest in clinical pharmacists who can contribute to reducing health care costs by preventing ADEs. Standardized methods of demonstrating the economic impact of clinical pharmacists’ interventions and how to value an ADE are needed to make reliable use of increasing amounts of electronic hospital data.
To consolidate these findings, it would be interesting to carry out a complete economic cost–benefit study, evaluating the cost impacts of long-term ADEs or assessing the savings that can be made through other patient-centred pharmaceutical interventions.
References
Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc. 2001;41:192–9. https://doi.org/10.1016/s1086-5802(16)31229-3.
Gyllensten H, Rehnberg C, Jönsson AK, et al. Cost of illness of patient-reported adverse drug events: a population-based cross-sectional survey. BMJ Open. 2013;3:e002574. https://doi.org/10.1136/bmjopen-2013-002574.
Rottenkolber D, Hasford J, Stausberg J. Costs of adverse drug events in German Hospitals—a microcosting study. Value Health. 2012;15:868–75. https://doi.org/10.1016/j.jval.2012.05.007.
Shehab N, Lovegrove MC, Geller AI, et al. US emergency department visits for outpatient adverse drug events, 2013–2014. JAMA. 2016;316:2115–25. https://doi.org/10.1001/jama.2016.16201.
Hardmeier B, Braunschweig S, Cavallaro M, et al. Adverse drug events caused by medication errors in medical inpatients. Swiss Med Wkly. 2004;134:664–70. https://doi.org/10.4414/smw.2004.10801.
Stausberg J. International prevalence of adverse drug events in hospitals: an analysis of routine data from England, Germany, and the USA. BMC Health Serv Res. 2014;14:125. https://doi.org/10.1186/1472-6963-14-125.
Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166:565–71. https://doi.org/10.1001/archinte.166.5.565.
Chen C-C, Hsiao F-Y, Shen L-J, et al. The cost-saving effect and prevention of medication errors by clinical pharmacist intervention in a nephrology unit. Medicine (Baltimore). 2017;96:e7883. https://doi.org/10.1097/MD.0000000000007883.
Chen P-Z, Wu C-C, Huang C-F. Clinical and economic impact of clinical pharmacist intervention in a hematology unit. J Oncol Pharm Pract. 2020;26:866–72. https://doi.org/10.1177/1078155219875806.
Dawoud DM, Smyth M, Ashe J, et al. Effectiveness and cost effectiveness of pharmacist input at the ward level: a systematic review and meta-analysis. Res Soc Adm Pharm. 2019;15:1212–22. https://doi.org/10.1016/j.sapharm.2018.10.006.
English S, Hort A, Sullivan N, et al. Is ward round participation by clinical pharmacists a valuable use of time and money? A time and motion study. Res Soc Adm Pharm. 2020;16:1026–32. https://doi.org/10.1016/j.sapharm.2019.10.014.
Gunterus A, Lopchuk S, Dunn C, et al. Quantitative and economic analysis of clinical pharmacist interventions during rounds in an acute care psychiatric hospital. Ment Health Clin. 2016;6:242–7. https://doi.org/10.9740/mhc.2016.09.242.
Rodrigues CR, Harrington AR, Murdock N, et al. Effect of pharmacy-supported transition-of-care interventions on 30-day readmissions: a systematic review and meta-analysis. Ann Pharmacother. 2017;51:866–89. https://doi.org/10.1177/1060028017712725.
Bethishou L, Herzik K, Fang N, et al. The impact of the pharmacist on continuity of care during transitions of care: a systematic review. J Am Pharm Assoc. 2020;60:163-177.e2. https://doi.org/10.1016/j.japh.2019.06.020.
Schumock GT, Meek PD, Ploetz PA, et al. Economic evaluations of clinical pharmacy services—1988–1995. Pharmacother J Hum Pharmacol Drug Ther. 1996;16:1188–208. https://doi.org/10.1002/j.1875-9114.1996.tb03049.x.
Schumock GT, Butler MG, Meek PD, et al. Evidence of the economic benefit of clinical pharmacy services: 1996–2000. Pharmacother J Hum Pharmacol Drug Ther. 2003;23:113–32. https://doi.org/10.1592/phco.23.1.113.31910.
Perez A, Doloresco F, Hoffman JM, et al. Economic evaluations of clinical pharmacy services: 2001–2005. Pharmacother J Hum Pharmacol Drug Ther. 2009;29:128–128. https://doi.org/10.1592/phco.29.1.128.
Touchette DR, Doloresco F, Suda KJ, et al. Economic evaluations of clinical pharmacy services: 2006–2010. Pharmacother J Hum Pharmacol Drug Ther. 2014;34:771–93. https://doi.org/10.1002/phar.1414.
Gallagher J, McCarthy S, Byrne S. Economic evaluations of clinical pharmacist interventions on hospital inpatients: a systematic review of recent literature. Int J Clin Pharm. 2014;36:1101–14. https://doi.org/10.1007/s11096-014-0008-9.
Mutnick AH, Sterba KJ, Peroutka JA, et al. Cost savings and avoidance from clinical interventions. Am J Health Syst Pharm. 1997;54:392–6.
Nesbit TW, Shermock KM, Bobek MB, et al. Implementation and pharmacoeconomic analysis of a clinical staff pharmacist practice model. Am J Health Syst Pharm. 2001;58:784–90.
Bosma BE. Pharmacist interventions during patient rounds in two intensive care units: clinical and financial impact. Neth J Med. 2018;76:10.
Jacob S, Britt RB, Bryan WE, et al. Economic outcomes associated with safety interventions by a pharmacist-adjudicated prior authorization consult service. J Manag Care Spec Pharm. 2019;25:411–6. https://doi.org/10.18553/jmcp.2019.25.3.411.
Simoens S, Jacobs I, Popovian R, et al. Assessing the value of biosimilars: a review of the role of budget impact analysis. Pharmacoeconomics. 2017;35:1047–62. https://doi.org/10.1007/s40273-017-0529-x.
Bonnabry P, François O. Return on investment: a practical calculation tool to convince your institution. Eur J Hosp Pharm. 2020;27:111–3. https://doi.org/10.1136/ejhpharm-2018-001733.
Berdot S, Blanc C, Chevalier D, et al. Impact of drug storage systems: a quasi-experimental study with and without an automated-drug dispensing cabinet. Int J Qual Health Care. 2019;31:225–30. https://doi.org/10.1093/intqhc/mzy155.
de Carvalho D, Alvim-Borges JL, Toscano CM. Impact assessment of an automated drug-dispensing system in a tertiary hospital. Clinics. 2017;72:629–36. https://doi.org/10.6061/clinics/2017(10)07.
Risør BW, Lisby M, Sørensen J. Cost-effectiveness analysis of an automated medication system implemented in a Danish Hospital setting. Value Health. 2017;20:886–93. https://doi.org/10.1016/j.jval.2017.03.001.
Claus BOM, Robays H, Decruyenaere J, et al. Expected net benefit of clinical pharmacy in intensive care medicine: a randomized interventional comparative trial with matched before-and-after groups. J Eval Clin Pract. 2014;20:1172–9. https://doi.org/10.1111/jep.12289.
Kopp BJ, Mrsan M, Erstad BL, et al. Cost implications of and potential adverse events prevented by interventions of a critical care pharmacist. Am J Health Syst Pharm. 2007;64:2483–7. https://doi.org/10.2146/ajhp060674.
Lee AJ, Boro MS, Knapp KK, et al. Clinical and economic outcomes of pharmacist recommendations in a Veterans Affairs medical center. Am J Health Syst Pharm. 2002;59:2070–7.
Benrimoj SI, Langford JH, Berry G, et al. Economic impact of increased clinical intervention rates in community pharmacy. Pharmacoeconomics. 2000;18:459–68. https://doi.org/10.2165/00019053-200018050-00005.
Truong H-A, Nicole Groves C, Congdon HB, et al. Potential cost savings of medication therapy management in safety-net clinics. J Am Pharm Assoc. 2015;55:269–72. https://doi.org/10.1331/JAPhA.2015.14062.
Randolph LA, Walker CK, Nguyen AT, et al. Impact of pharmacist interventions on cost avoidance in an ambulatory cancer center. J Oncol Pharm Pract. 2018;24:3–8. https://doi.org/10.1177/1078155216671189.
Chinthammit C, Armstrong EP, Warholak TL. A cost-effectiveness evaluation of hospital discharge counseling by pharmacists. J Pharm Pract. 2012;25:201–8. https://doi.org/10.1177/0897190011418512.
Jourdan J-P, Muzard A, Goyer I, et al. Impact of pharmacist interventions on clinical outcome and cost avoidance in a university teaching hospital. Int J Clin Pharm. 2018;40:1474–81. https://doi.org/10.1007/s11096-018-0733-6.
Ahmad A, Weston PJ, Ahmad M, et al. A cost-benefit analysis of twice-daily consultant ward rounds and clinical input on investigation and pharmacy costs in a major teaching hospital in the UK. BMJ Open. 2015;5:e007367. https://doi.org/10.1136/bmjopen-2014-007367.
Yasunaga D, Tasaka Y, Murakami S, et al. Economic contributions of pharmaceutical interventions by pharmacists: a retrospective report in Japan. J Pharm Policy Pract. 2016;10:2. https://doi.org/10.1186/s40545-016-0073-7.
Saokaew S, Maphanta S, Thangsomboon P. Impact of pharmacist’s interventions on cost of drug therapy in intensive care unit. Pharm Pract. 2009;7:81–7. https://doi.org/10.4321/s1886-36552009000200003.
Bao Z, Ji C, Hu J, et al. Clinical and economic impact of pharmacist interventions on sampled outpatient prescriptions in a Chinese teaching hospital. BMC Health Serv Res. 2018;18:519. https://doi.org/10.1186/s12913-018-3306-4.
Tasaka Y, Tanaka A, Yasunaga D, et al. Potential drug-related problems detected by routine pharmaceutical interventions: safety and economic contributions made by hospital pharmacists in Japan. J Pharm Health Care Sci. 2018;4:33. https://doi.org/10.1186/s40780-018-0125-z.
Tasaka Y, Yasunaga D, Tanaka M, et al. Economic and safety benefits of pharmaceutical interventions by community and hospital pharmacists in Japan. Int J Clin Pharm. 2016;38:321–9. https://doi.org/10.1007/s11096-015-0245-6.
Griese-Mammen N, Hersberger KE, Messerli M, et al. PCNE definition of medication review: reaching agreement. Int J Clin Pharm. 2018;40:1199–208. https://doi.org/10.1007/s11096-018-0696-7.
Hepler CD, Strand LM. Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm. 1990;47:533–43. https://doi.org/10.1093/ajhp/47.3.533.
Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA. 1995;274:29–34. https://doi.org/10.1001/jama.1995.03530010043033.
Guignard B, Bonnabry P, Perrier A, et al. Drug-related problems identification in general internal medicine: the impact and role of the clinical pharmacist and pharmacologist. Eur J Intern Med. 2015;26:399–406. https://doi.org/10.1016/j.ejim.2015.05.012.
Procédure de certification REKOLE®. https://www.hplus.ch/fr/comptabilite/proceduredecertificationrekoler/. Accessed 6 Jan 2019.
World Health Organization International Classification of Diseases (ICD). https://www.who.int/standards/classifications/classification-of-diseases. Accessed 14 Sep 2023.
World Health Organization ICD Update Platform. https://icd.who.int/icd10updateplatform/. Accessed 14 May 2020.
Neville HL, Chevalier B, Daley C, et al. Clinical benefits and economic impact of post-surgical care provided by pharmacists in a Canadian hospital. Int J Pharm Pract. 2014;22:216–22. https://doi.org/10.1111/ijpp.12058.
Gallagher J, Byrne S, Woods N, et al. Cost-outcome description of clinical pharmacist interventions in a university teaching hospital. BMC Health Serv Res. 2014;14:177. https://doi.org/10.1186/1472-6963-14-177.
Gallagher J, O’Sullivan D, McCarthy S, et al. Structured pharmacist review of medication in older hospitalised patients: a cost-effectiveness analysis. Drugs Aging. 2016;33:285–94. https://doi.org/10.1007/s40266-016-0348-3.
Calloway S, Akilo HA, Bierman K. Impact of a clinical decision support system on pharmacy clinical interventions, documentation efforts, and costs. Hosp Pharm. 2013;48:744–52. https://doi.org/10.1310/hpj4809-744.
Lewkowicz D, Wohlbrandt A, Boettinger E. Economic impact of clinical decision support interventions based on electronic health records. BMC Health Serv Res. 2020;20:871. https://doi.org/10.1186/s12913-020-05688-3.
Skalafouris C, Samer C, Stirnemann J, et al. Electronic monitoring of potential adverse drug events related to lopinavir/ritonavir and hydroxychloroquine during the first wave of COVID-19. Eur J Hosp Pharm. 2021;30:113–6. https://doi.org/10.1136/ejhpharm-2020-002667.
D’hulster E, Quintens C, Bisschops R, et al. Cost-effectiveness of check of medication appropriateness: methodological approach. Int J Clin Pharm. 2022;44:399–408. https://doi.org/10.1007/s11096-021-01356-6.
Bosma LBE, Hunfeld NGM, Quax RAM, et al. The effect of a medication reconciliation program in two intensive care units in the Netherlands: a prospective intervention study with a before and after design. Ann Intensive Care. 2018;8:19. https://doi.org/10.1186/s13613-018-0361-2.
Sjölander M, Lindholm L, Pfister B, et al. Impact of clinical pharmacist engagement in ward teams on the number of drug-related readmissions among older patients with dementia or cognitive impairment: an economic evaluation. Res Soc Adm Pharm. 2019;15:287–91. https://doi.org/10.1016/j.sapharm.2018.05.006.
Odeh M, Scullin C, Hogg A, et al. A novel approach to medicines optimisation post-discharge from hospital: pharmacist-led medicines optimisation clinic. Int J Clin Pharm. 2020;42:1036–49. https://doi.org/10.1007/s11096-020-01059-4.
Al-Qudah RA, Al-Badriyeh D, Al-Ali FM, et al. Cost-benefit analysis of clinical pharmacist intervention in preventing adverse drug events in the general chronic diseases outpatients. J Eval Clin Pract. 2020;26:115–24. https://doi.org/10.1111/jep.13209.
Wallerstedt SM, Bladh L, Ramsberg J. A cost-effectiveness analysis of an in-hospital clinical pharmacist service. BMJ Open. 2012;2:e000329. https://doi.org/10.1136/bmjopen-2011-000329.
Avery AJ, Rodgers S, Cantrill JA, et al. A pharmacist-led information technology intervention for medication errors (PINCER): a multicentre, cluster randomised, controlled trial and cost-effectiveness analysis. The Lancet. 2012;379:1310–9. https://doi.org/10.1016/S0140-6736(11)61817-5.
Karnon J, Campbell F, Czoski-Murray C. Model-based cost-effectiveness analysis of interventions aimed at preventing medication error at hospital admission (medicines reconciliation). J Eval Clin Pract. 2009;15:299–306. https://doi.org/10.1111/j.1365-2753.2008.01000.x.
Twigg MJ, Wright D, Barton G, et al. The pharmacy care plan service: evaluation and estimate of cost-effectiveness. Res Soc Adm Pharm. 2019;15:84–92. https://doi.org/10.1016/j.sapharm.2018.03.062.
Jódar-Sánchez F, Malet-Larrea A, Martín JJ, et al. Cost-utility analysis of a medication review with follow-up service for older adults with polypharmacy in community pharmacies in Spain: the conSIGUE program. Pharmacoeconomics. 2015;33:599–610. https://doi.org/10.1007/s40273-015-0270-2.
Malet-Larrea A, Goyenechea E, Gastelurrutia MA, et al. Cost analysis and cost-benefit analysis of a medication review with follow-up service in aged polypharmacy patients. Eur J Health Econ. 2017;18:1069–78. https://doi.org/10.1007/s10198-016-0853-7.
Elliott RA, Lee CY, Beanland C, et al. Development of a clinical pharmacy model within an Australian home nursing service using co-creation and participatory action research: the Visiting Pharmacist (ViP) study. BMJ Open. 2017;7:e018722. https://doi.org/10.1136/bmjopen-2017-018722.
Karapinar-Çarkit F, Borgsteede SD, Zoer J, et al. Effect of medication reconciliation on medication costs after hospital discharge in relation to hospital pharmacy labor costs. Ann Pharmacother. 2012;46:329–38. https://doi.org/10.1345/aph.1Q520.
Malone DC, Carter BL, Billups SJ, et al. An economic analysis of a randomized, controlled, multicenter study of clinical pharmacist interventions for high-risk veterans: the IMPROVE study. Pharmacother J Hum Pharmacol Drug Ther. 2012;20:1149–58. https://doi.org/10.1592/phco.20.15.1149.34590.
Hung P-L, Chen J-Y, Chen M-T, et al. The impact of a medication reconciliation programme at geriatric hospital admission: a pre-/postintervention study. Br J Clin Pharmacol. 2019;85:2614–22. https://doi.org/10.1111/bcp.14095.
Weant KA, Armitstead JA, Ladha AM, et al. Cost effectiveness of a clinical pharmacist on a neurosurgical team. Neurosurgery. 2009;65:946–51. https://doi.org/10.1227/01.NEU.0000347090.22818.35.
Acknowledgements
Copyediting, English proofreading and editorial assistance were provided by Mr. Darren Hart of Publish or Perish (Nyon, Switzerland) and AJE Part of Springer Nature (USA).
Funding
Open access funding provided by University of Geneva. No specific funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jermini, M., Fonzo-Christe, C., Blondon, K. et al. Financial impact of medication reviews by clinical pharmacists to reduce in-hospital adverse drug events: a return-on-investment analysis. Int J Clin Pharm 46, 496–505 (2024). https://doi.org/10.1007/s11096-023-01683-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-023-01683-w