Abstract
Background
A six year collaboration between academics, community pharmacists and informaticians, led to the development of nine guidelines for a clinical decision support system, enhancing community pharmacists’ ability to address drug-related problems and improve care.
Aim
The objective of this study was to assess the effectiveness of clinical decision support system rules in enhancing medication management within the community pharmacy setting. This was achieved through retrospective monitoring of real-world usage and measuring the pharmacotherapeutic impact of the rules.
Method
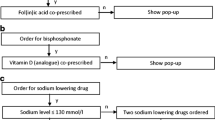
In 2019, a retrospective observational evaluation appraised the acceptance rate of the clinical decision support system components in 490 Belgian pharmacies. Among these, 51 pharmacies underwent a longitudinal analysis involving (i) co-prescription of methotrexate and folic acid, (ii) gastroprotection with non-steroidal anti-inflammatory drugs, and (iii) drug combinations causing QT prolongation. The study period spanned one year pre-launch, one year post-launch, and two years post-launch.
Results
Of the targeted pharmacies, 80% used 7 of the 9 rules. After four years, methotrexate-folic acid co-prescription increased 4%, reaching 79.8%. Gastroprotection improved by 3% among older patients and 7.47% in younger individuals (< 70 year) with multiple risk factors. The QT prolongation rules faced implementation difficulties.
Conclusion
Pharmacists’ acceptance of the developed rules was high and coincided with a decline in drug-related problems, holding potential public health impact. This real-world data can inform the future implementation of such systems, as it demonstrated the need for more detailed data-gathering and more intensive training of pharmacists in the handling of more complex problems such as QT prolongation.


Active: the pop-up alert of this topic is activated and being used


Similar content being viewed by others
References
Leendertse AJ, Egberts ACG, Stoker LJ, et al. Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. Arch Intern Med. 2008;168(17):1890–6. https://doi.org/10.1001/archinternmed.2008.3.
IMS_Health Report. Advancing the responsible use of medicines in Belgium. 2015. Available from: https://amcham.app.box.com/s/wufmzyqimb4d0ytbyayqoejs3h6ams5e. Accessed 02 Dec 2022.
Van den Bemt PM, de Smet PM, Hek K, et al. Eindrapport: Vervolgonderzoek Medicatieveiligheid. Erasmus MC, NIVEL, Radboud UMC. Vervolgonderzoek medicatieveiligheid: eindrapport. 2017. Available from: https://www.nivel.nl/sites/default/files/bestanden/Vervolgonderzoek_Medicatieveiligheid_Eindrapport.pdf. Accessed 02 Dec 2022.
Saedder EA, Brock B, Nielsen LP, et al. Identifying high-risk medication: a systematic literature review. Eur J Clin Pharmacol. 2014;70(6):637–45. https://doi.org/10.1007/S00228-014-1668-Z.
Hämmerlein A, Griese N, Schulz M. Survey of drug-related problems identified by community pharmacies. Ann Pharmacother. 2007;41(11):1825–32. https://doi.org/10.1345/aph.1k207.
Reis WC, Bonetti AF, Bottacin WE, et al. Impact on process results of clinical decision support systems (CDSSs) applied to medication use: overview of systematic reviews. Pharm Pract (Granada). 2017. https://doi.org/10.18549/PHARMPRACT.2017.04.1036.
Osheroff J, Teich JM, Levick D, et al. Improving outcomes with clinical decision support: an implementer’s guide. 2nd Edition. Chicago: CRC Press. 2012; p. 323. ISBN: 9780984457731.
Watkins K, Wood H, Schneider CR, et al. Effectiveness of implementation strategies for clinical guidelines to community pharmacy: a systematic review. Implement Sci. 2015. https://doi.org/10.1186/S13012-015-0337-7.
Luyssen J, Wauters M, Moerman H, et al. Evaluation of an electronic decision support program for optimization of the medication schedule in ambulatory elderly. Tijdschr Geneeskd. 2019;75(8):622–31.
Mulder-Wildemors LGM, Heringa M, Floor-Schreudering A, et al. Reducing inappropriate drug use in older patients by use of clinical decision support in community pharmacy: a mixed-methods evaluation. Drugs Aging. 2020;37(2):115–23. https://doi.org/10.1007/S40266-019-00728-Y.
Sönnichsen A, Trampisch US, Rieckert A, et al. Polypharmacy in chronic diseases-reduction of inappropriate medication and adverse drug events in older populations by electronic decision support (PRIMA-eDS): study protocol for a randomized controlled trial. Trials. 2016. https://doi.org/10.1186/S13063-016-1177-8.
Wasylewicz A, Scheepers-Hoeks A. Clinical decision support systems. In: Kubben P, Dumontier M, Dekker A, editors. Fundamentals of clinical data science. Cham: Springer; 2019. p. 153–69.
Schillemans S, de Loof H, de Meyer G. Kritische evaluatie van geneesmiddelen- interactiesoftware in de apotheek. Farmaceutisch tijdschrift voor België. 2012;89(4):4–13.
Claudot F, Alla F, Fresson J, et al. Ethics and observational studies in medical research: various rules in a common framework. Int J Epidemiol. 2009;38(4):1104. https://doi.org/10.1093/IJE/DYP164.
Curtain C, Peterson G. Review of computerized clinical decision support in community pharmacy. J Clin Pharm Ther. 2014;39(4):343–8. https://doi.org/10.1111/JCPT.12168.
Shahmoradi L, Safdari R, Ahmadi H, et al. Clinical decision support systems-based interventions to improve medication outcomes: a systematic literature review on features and effects. Med J Islam Repub Iran. 2021. https://doi.org/10.47176/MJIRI.35.27.
Verduijn MM, Van den Bemt BJF, Dijkmans BAC, et al. Methotrexaat veilig, mits juist voorgeschreven. Ned Tijdschr Geneeskd. 2009;153:A695. Available from: https://www.ntvg.nl/artikelen/methotrexaat-veilig-mits-juist-voorgeschreven. Accessed 02 Dec 2022.
Maagklachten. NHG-Richtlijnen. 2021. Available from: https://richtlijnen.nhg.org/standaarden/maagklachten. Accessed 07 Dec 2022.
Crediblemeds. Available from: https://crediblemeds.org/. Accessed 02 Dec 2022.
Drew BJ, Ackerman MJ, Funk M, et al. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2010;55(9):934–47. https://doi.org/10.1016/J.JACC.2010.01.001.
Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350(10):1013–22. https://doi.org/10.1056/NEJMRA032426.
van Noord C, Eijgelsheim M, Stricker BHC. Drug- and non-drug-associated QT interval prolongation. Br J Clin Pharmacol. 2010;70(1):16–23. https://doi.org/10.1111/J.1365-2125.2010.03660.X.
Vandael E, Vandenberk B, Vandenberghe J, et al. Development of a risk score for QTc-prolongation: the RISQ-PATH study. Int J Clin Pharm. 2017;39(2):424–32. https://doi.org/10.1007/S11096-017-0446-2.
Sutton RT, Pincock D, Baumgart DC, et al. An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit Med. 2020;3(1):1–10. https://doi.org/10.1038/s41746-020-0221-y.
Mazaud C, Fardet L. Folate supplementation during methotrexate therapy: a population-based retrospective cohort study. Acta Derm Venereol. 2018;98(5):536–8. https://doi.org/10.2340/00015555-2904.
SFK Website. Available from: https://www.sfk.nl/. Accessed 02 Dec 2022.
RIZIV/INAMI. Farmaceutische kengetallen Farmaceutische verstrekkingen Ambulante praktijk Comité voor de evaluatie van de medische praktijk inzake geneesmiddelen. 2014. Available from: https://www.inami.fgov.be/SiteCollectionDocuments/farma_kengetallen_verslag_2014.pdf. Accessed 08 Dec 2022.
Bollen A, Harrison R, Aslani P, et al. Factors influencing interprofessional collaboration between community pharmacists and general practitioners-A systematic review. Health Soc Care Commun. 2019;27(4):E189-212. https://doi.org/10.1111/HSC.12705.
BCFI. Available from: https://www.bcfi.be/nl/start Accessed 02 Dec 2022.
G-Standaard—Z-Index. Available from: https://www.g-standaard.nl/%20risanalysis/M0000107.pdf. Accessed 02 Dec 2022.
Nederlandse Vereniging voor Reumatologie. Osteoporose en fractuurpreventie, derde herziening. Utrecht; 2011. Available from: https://ephor.nl/wp-content/uploads/2018/12/richtlijn-osteoporose-28-04-2011.pdf. Accessed 07 Dec 2022.
Fracture Risk Assessment Tool (FRAX): What it is, how it works, and more. Available from: https://www.webmd.com/osteoporosis/fracture-risk-assessment-tool. Accessed 08 Dec 2022.
Hoge Gezondheidsraad. Advies 9531: vaccinatie tegen griep|FOD Volksgezondheid. 2019. Available from: https://www.health.belgium.be/nl/advies-9531-vaccinatie-tegen-griep. Accessed 02 Dec 2022.
Acknowledgements
The authors would like to thank Frederic Vancraeyveldt for the support in providing the analysis data and Bronwen Martin for her critical reading of the manuscript. Royal Society of Pharmacists of East Flanders (KOVAG) and University of Antwerp.
Funding
No specific funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bogaerts, C., Schoenmaekers, N., Haems, M. et al. A quality improvement study of the implementation and initial results of a pragmatic clinical decision support system in the community pharmacy setting. Int J Clin Pharm 46, 141–149 (2024). https://doi.org/10.1007/s11096-023-01648-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-023-01648-z