Abstract
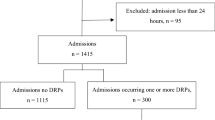
Background Geriatric patients represent a vulnerable population in terms of adverse drug events (ADEs). Objective The aims of this study were to determine the prevalence and preventability of hospital admissions to a geriatric ward related to ADEs, to identify medications involved in these ADEs and to describe potential preventability aspects of ADE-related admissions. Setting University Hospital Hradec Králové, Czech Republic. Methods This cross-sectional study evaluated acute hospital admissions to the geriatric ward of University Hospital Hradec Králové over a period of nine months (April–December 2017). Medication reviews were performed in order to identify ADE-related hospital admissions. Causality was assessed using the World Health Organization-Uppsala Monitoring Centre criteria. Modified Schumock-Thornton algorithm was used to assess the preventability of ADEs. Main outcome measure 9-month-prevalence of ADE-related hospital admissions. Results A total of 366 hospital admissions were included. The 9-month-prevalence of ADE-related hospital admissions was 11.75% [95% confidence interval 8.45–15.05]. Antithrombotic agents and diuretics represented the most common medication classes associated with ADEs (30.2% each). Electrolyte disturbances and gastrointestinal haemorrhages and ulcerations were the most frequently observed ADEs associated with hospital admission. Out of 43 ADE-related hospitalisations, 23 (53.5%) were considered potentially preventable. Conclusion The contribution of ADEs to hospital admission to the geriatric ward was not negligible. Our results also suggest that 53.5% of identified ADE-related admissions could be potentially prevented. This finding demonstrates just how important the research on the preventability of medication-related hospitalisations is. Further studies and implementations are still needed aiming to minimize the risk of medication-related harm.
Similar content being viewed by others
References
Medication Without Harm-Global Patient Safety Challenge on Medication Safety. World Health Organization, Geneva. 2017. https://www.who.int/patientsafety/medication-safety/en/. Accessed 25 Nov 2020.
Griese-Mammen N, Hersberger KE, Messerli M, Leikola S, Horvat N, van Mil JWF, et al. PCNE definition of medication review: reaching agreement. Int J Clin Pharm. 2018;40(5):1199–208.
Jolivot PA, Pichereau C, Hindlet P, Hejblum G, Bige N, Maury E et al. An observational study of adult admissions to a medical ICU due to adverse drug events. Ann Intensiv Care. 2016;6(1):1–12.
Linkens A, Milosevic V, van der Kuy PHM, Damen-Hendriks VH, Mestres Gonzalvo C, Hurkens K. Medication-related hospital admissions and readmissions in older patients: an overview of literature. Int J Clin Pharm. 2020;5:10.
Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: a clinician’s guide to terminology, documentation, and reporting. Ann Intern Med. 2004;140(10):795–801.
van Foppe MJW, Westerlund T, Brown L, Chen TF, Henman M, Hersberger K, et al. Medical care and drug-related problems: Do doctors and pharmacists speak the same language? Int J Clin Pharm. 2016;38(2):191–4.
Langerova P, Vrtal J, Urbanek K. Adverse drug reactions causing hospital admissions in childhood: a prospective, observational. Single-Centre Study Basic Clin Pharmacol Toxicol. 2014;115(6):560–4.
Leendertse AJ, Visser D, Egberts AC, van den Bemt PM. The relationship between study characteristics and the prevalence of medication-related hospitalizations: a literature review and novel analysis. Drug Saf. 2010;33(3):233–44.
Beijer HJM, de Blaey CJ. Hospitalisations caused by adverse drug reactions (ADR): a meta-analysis of observational studies. Pharm World Sci. 2002;24(2):46–54.
The use of the WHO-UMC system for standardised case causality assessment. https://www.who.int/medicines/areas/quality_safety/safety_efficacy/WHOcausality_assessment.pdf. Accessed 25 Nov 2020.
Schmiedl S, Rottenkolber M, Szymanski J, Drewelow B, Siegmund W, Hippius M, et al. Preventable ADRs leading to hospitalization-results of a long-term prospective safety study with 6,427 ADR cases focusing on elderly patients. Expert Opin Drug Saf. 2018;17(2):125–37.
Parameswaran Nair N, Chalmers L, Peterson GM, Bereznicki BJ, Curtain CM, Bereznicki LR. Prospective identification versus administrative coding of adverse drug reaction-related hospitalizations in the elderly: a comparative analysis. Pharmacoepidemiol Drug Saf. 2018;27(11):1281–5.
Petrovic M, van der Cammen T, Onder G. Adverse drug reactions in older people: detection and prevention. Drugs Aging. 2012;29(6):453–62.
Conforti A, Costantini D, Zanetti F, Moretti U, Grezzana M, Leone R. Adverse drug reactions in older patients: an Italian observational prospective hospital study. Drug Healthc Patient Saf. 2012;4:75–80.
Oscanoa TJ, Lizaraso F, Carvajal A. Hospital admissions due to adverse drug reactions in the elderly. A meta-analysis. Eur J Clin Pharmacol. 2017;73(6):759–70.
Laatikainen O, Sneck S, Bloigu R, Lahtinen M, Lauri T, Turpeinen M. Hospitalizations due to adverse drug events in the elderly-a retrospective register study. Front Pharmacol. 2016;7(358):697.
Thevelin S, Spinewine A, Beuscart JB, Boland B, Marien S, Vaillant F, et al. Development of a standardized chart review method to identify drug-related hospital admissions in older people. Br J Clin Pharmacol. 2018;84(11):2600–14.
Kempen TGH, Hedström M, Olsson H, Johansson A, Ottosson S, Al-Sammak Y, et al. Assessment tool for hospital admissions related to medications: development and validation in older patients. Int J Clin Pharm. 2019;41(1):198–206.
Wawruch M, Zikavska M, Wsolova L, Kuzelova M, Kahayova K, Strateny K, et al. Adverse drug reactions related to hospital admission in Slovak elderly patients. Arch Gerontol Geriatr. 2009;48(2):186–90.
Franceschi M, Scarcelli C, Niro V, Seripa D, Pazienza AM, Pepe G, et al. Prevalence, clinical features and avoidability of adverse drug reactions as cause of admission to a Geriatric Unit-a prospective study of 1756 patients. Drug Saf. 2008;31(6):545–56.
Nair NP, Chalmers L, Bereznicki BJ, Curtain C, Peterson GM, Connolly M, et al. Adverse drug reaction-related hospitalizations in elderly australians: a prospective cross-sectional study in two tasmanian hospitals. Drug Saf. 2017;40(7):597–606.
Ognibene S, Vazzana N, Giumelli C, Savoldi L, Braglia L, Chesi G. Hospitalisation and morbidity due to adverse drug reactions in elderly patients: a single-centre study. Intern Med J. 2018;48(10):1192–7.
Rogers S, Wilson D, Wan S, Griffin M, Rai G, Farrell J. Medication-related admissions in older people a cross-sectional. Observ Study Drugs Aging. 2009;26(11):951–61.
Onder G, Pedone C, Landi F, Cesari M, Della Vedova C, Bernabei R, et al. Adverse drug reactions as cause of hospital admissions: results from the Italian Group of Pharmacoepidemiology in the Elderly (GIFA). J Am Geriatr Soc. 2002;50(12):1962–8.
Olivier P, Bertrand L, Tubery M, Lauque D, Montastruc JL, Lapeyre-Mestre M. Hospitalizations because of adverse drug reactions in elderly patients admitted through the emergency department a prospective survey. Drugs Aging. 2009;26(6):475–82.
Bayoumi I, Dolovich L, Hutchison B, Holbrook A. Medication-related emergency department visits and hospitalizations among older adults. Can Fam Physician. 2014;60(4):e217–22.
Pedros C, Formiga F, Corbella X, Arnau JM. Adverse drug reactions leading to urgent hospital admission in an elderly population: prevalence and main features. Eur J Clin Pharmacol. 2016;72(2):219–26.
Ruiter R, Visser LE, Rodenburg EM, Trifir G, Ziere G, Stricker BH. Adverse drug reaction-related hospitalizations in persons aged 55 years and over: a population-based study in the Netherlands. Drugs Aging. 2012;29(3):225–32.
Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002–12.
Cabre M, Elias L, Garcia M, Palomera E, Serra-Prat M. Avoidable hospitalizations due to adverse drug reactions in an acute geriatric unit. Analysis of 3292 patients. Med Clin (Barc). 2018;150(6):209–14.
Passarelli MCG, Jacob W, Figueras A. Adverse drug reactions in an elderly hospitalised population-inappropriate prescription is a leading cause. Drugs Aging. 2005;22(9):767–77.
Marcum ZA, Amuan ME, Hanlon JT, Aspinall SL, Handler SM, Ruby CM, et al. Prevalence of unplanned hospitalizations caused by adverse drug reactions in older veterans. J Am Geriatr Soc. 2012;60(1):34–41.
Wojszel ZB, Kasiukiewicz A. A retrospective cross-sectional study of type 2 diabetes overtreatment in patients admitted to the geriatric ward. BMC Geriatr. 2019;19(1):242.
Acknowledgments
This study was supported by Charles University (Project SVV 260 551).
Funding
This study was supported by Charles University (Project SVV 260 551).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics Aprroval
This study was approved by University Hospital Hradec Králové (number of the ethics approval letter 201701S13R). Patient informed consent was not required due to the observational design of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maříková, M., Očovská, Z., Nerad, V. et al. Hospital admissions to geriatric ward related to adverse drug events: a cross-sectional study from the Czech Republic. Int J Clin Pharm 43, 1218–1226 (2021). https://doi.org/10.1007/s11096-021-01237-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-021-01237-y