Abstract
Purpose
The physiologically based pharmacokinetic (PBPK) modeling has received increasing attention owing to its excellent predictive abilities. However, there has been no bibliometric analysis about PBPK modeling. This research aimed to summarize the research development and hot points in PBPK model utilization overall through bibliometric analysis.
Methods
We searched for publications related to the PBPK modeling from 1999 to 2023 in the Web of Science Core Collection (WoSCC) database. The Microsoft Office Excel, CiteSpace and VOSviewers were used to perform the analyses.
Results
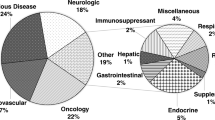
A total of 4,649 records from 1999 to 2023 were identified, and the largest number of publications focused in the period 2018–2023. The United States was the leading country, and the Environmental Protection Agency (EPA) was the leading institution. The journal Drug Metabolism and Disposition published and co-cited the most articles. Drug–drug interactions, special populations, and new drug development are the main topics in this research field.
Conclusion
We first visualize the research landscape and hotspots of the PBPK modeling through bibliometric methods. Our study provides a better understanding for researchers, especially beginners about the dynamization of PBPK modeling and presents the relevant trend in the future.









Similar content being viewed by others
Data Availability
The original contributions presented in the study are included in the article material. Further inquiries can be directed to the corresponding author.
References
Utembe W, Clewell H, Sanabria N, Doganis P, Gulumian M. Current approaches and techniques in physiologically based pharmacokinetic (PBPK) modelling of nanomaterials. Nanomaterials (Basel). 2020;10(7):1267.
Jones H, Rowland-Yeo K. Basic concepts in physiologically based pharmacokinetic modeling in drug discovery and development. CPT Pharmacometrics Syst Pharmacol. 2013;2(8):e63.
Peters SA, Dolgos H. Requirements to establishing confidence in physiologically based pharmacokinetic (PBPK) models and overcoming some of the challenges to meeting them. Clin Pharmacokinet. 2019;58(11):1355–71.
Wu F, Zhou Y, Li L, Shen X, Chen G, Wang X, et al. Computational approaches in preclinical studies on drug discovery and development. Front Chem. 2020;8:726.
Polak S, Tylutki Z, Holbrook M, Wisniowska B. Better prediction of the local concentration-effect relationship: the role of physiologically based pharmacokinetics and quantitative systems pharmacology and toxicology in the evolution of model-informed drug discovery and development. Drug Discov Today. 2019;24(7):1344–54.
Zhu Q, Chen Z, Paul PK, Lu Y, Wu W, Qi J. Oral delivery of proteins and peptides: Challenges, status quo and future perspectives. Acta Pharm Sin B. 2021;11(8):2416–48.
Traber GM, Yu AM. RNAi-based therapeutics and novel RNA bioengineering technologies. J Pharmacol Exp Ther. 2023;384(1):133–54.
Smits A, De Cock P, Vermeulen A, Allegaert K. Physiologically based pharmacokinetic (PBPK) modeling and simulation in neonatal drug development: how clinicians can contribute. Expert Opin Drug Metab Toxicol. 2019;15(1):25–34.
Verscheijden LFM, Koenderink JB, Johnson TN, de Wildt SN, Russel FGM. Physiologically-based pharmacokinetic models for children: Starting to reach maturation? Pharmacol Ther. 2020;211:107541.
Fairman K, Choi MK, Gonnabathula P, Lumen A, Worth A, Paini A, et al. An overview of physiologically-based pharmacokinetic models for forensic science. Toxics. 2023;11(2):126.
Lin W, Chen Y, Unadkat JD, Zhang X, Wu D, Heimbach T. Applications, challenges, and outlook for PBPK modeling and simulation: A Regulatory, industrial and academic perspective. Pharm Res. 2022;39(8):1701–31.
Tsakalozou E, Alam K, Babiskin A, Zhao L. Physiologically-based pharmacokinetic modeling to support determination of bioequivalence for dermatological drug products: scientific and regulatory considerations. Clin Pharmacol Ther. 2022;111(5):1036–49.
Anand O, Pepin XJH, Kolhatkar V, Seo P. The use of physiologically based pharmacokinetic analyses-in biopharmaceutics applications -regulatory and industry perspectives. Pharm Res. 2022;39(8):1681–700.
Wang B, Xing D, Zhu Y, Dong S, Zhao B. The state of exosomes research: A global visualized analysis. Biomed Res Int. 2019;2019:1495130.
Poletto VC, Faraco Junior IM. Bibliometric study of articles published in a Brazilian journal of pediatric dentistry. Braz Oral Res. 2010;24(1):83–8.
Chen CM, Ibekwe-SanJuan F, Hou JH. The structure and dynamics of cocitation clusters: A multiple-perspective cocitation analysis. J Am Soc Inf Sci Tec. 2010;61(7):1386–409.
Pei Z, Chen S, Ding L, Liu J, Cui X, Li F, et al. Current perspectives and trend of nanomedicine in cancer: A review and bibliometric analysis. J Control Release. 2022;352:211–41.
Chen P, Zhong C, Jin S, Zhang Y, Li Y, Xia Q, et al. Global trends in research of lipid metabolism in T lymphocytes from 1985 to 2022: A bibliometric analysis. Front Immunol. 2022;13:884030.
Chen C, Dubin R, Kim MC. Emerging trends and new developments in regenerative medicine: a scientometric update (2000–2014). Expert Opin Biol Ther. 2014;14(9):1295–317.
Chen CM. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Tec. 2006;57(3):359–77.
van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–38.
Bradford S. Sources of information on specific subjects. Engineering. 1934;137:85–6.
Venable GT, Shepherd BA, Loftis CM, McClatchy SG, Roberts ML, Fillinger ME, et al. Bradford’s law: identification of the core journals for neurosurgery and its subspecialties. J Neurosurg. 2016;124(2):569–79.
Egghe L. Applications of the theory of Bradford’s Law to the calculation of Leimkuhler’s Law and to the completion of bibliographies. J Am Soc Inf Sci. 1990;41:469–92.
Chen CM. Science mapping: A systematic review of the literature. J Data Inf Sci. 2017;2:1–40.
Zheng MQ, Li XX, Xu R, Liu S, Rui ZY, Guo ZY, et al. Bibliometric analysis of tuberculosis molecular epidemiology based on CiteSpace. Front Public Health. 2022;10:1040176.
Sabe M, Chen C, Perez N, Solmi M, Mucci A, Galderisi S, et al. Thirty years of research on negative symptoms of schizophrenia: A scientometric analysis of hotspots, bursts, and research trends. Neurosci Biobehav Rev. 2023;144:104979.
Rowland M, Peck C, Tucker G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol. 2011;51:45–73.
Shebley M, Sandhu P, EmamiRiedmaier A, Jamei M, Narayanan R, Patel A, et al. Physiologically based pharmacokinetic model qualification and reporting procedures for regulatory submissions: A consortium perspective. Clin Pharmacol Ther. 2018;104(1):88–110.
Jones HM, Chen Y, Gibson C, Heimbach T, Parrott N, Peters SA, et al. Physiologically based pharmacokinetic modeling in drug discovery and development: a pharmaceutical industry perspective. Clin Pharmacol Ther. 2015;97(3):247–62.
Grimstein M, Yang Y, Zhang X, Grillo J, Huang SM, Zineh I, et al. Physiologically based pharmacokinetic modeling in regulatory science: An update from the U.S. food and drug administration’s office of clinical pharmacology. J Pharm Sci. 2019;108(1):21–5.
Sager JE, Yu J, Ragueneau-Majlessi I, Isoherranen N. Physiologically Based Pharmacokinetic (PBPK) modeling and simulation approaches: A systematic review of published models, applications, and model verification. Drug Metab Dispos. 2015;43(11):1823–37.
Zhao P, Zhang L, Grillo JA, Liu Q, Bullock JM, Moon YJ, et al. Applications of physiologically based pharmacokinetic (PBPK) modeling and simulation during regulatory review. Clin Pharmacol Ther. 2011;89(2):259–67.
Wagner C, Zhao P, Pan Y, Hsu V, Grillo J, Huang SM, et al. Application of Physiologically Based Pharmacokinetic (PBPK) modeling to support dose selection: Report of an FDA public workshop on PBPK. CPT Pharmacometrics Syst Pharmacol. 2015;4(4):226–30.
Zhuang X, Lu C. PBPK modeling and simulation in drug research and development. Acta Pharm Sin B. 2016;6(5):430–40.
Jamei M. Recent advances in development and application of Physiologically-Based Pharmacokinetic (PBPK) Models: a Transition from Academic Curiosity to Regulatory Acceptance. Curr Pharmacol Rep. 2016;2(3):161–9.
Kuepfer L, Niederalt C, Wendl T, Schlender JF, Willmann S, Lippert J, et al. Applied concepts in PBPK modeling: How to build a PBPK/PD model. CPT Pharmacometrics Syst Pharmacol. 2016;5(10):516–31.
Peng C, Kuang L, Zhao J, Ross AE, Wang Z, Ciolino JB. Bibliometric and visualized analysis of ocular drug delivery from 2001 to 2020. J Control Release. 2022;345:625–45.
Chen C, Hu Z, Liu S, Tseng H. Emerging trends in regenerative medicine: a scientometric analysis in CiteSpace. Expert Opin Biol Ther. 2012;12(5):593–608.
Qin YF, Ren SH, Shao B, Qin H, Wang HD, Li GM, et al. The intellectual base and research fronts of IL-37: A bibliometric review of the literature from WoSCC. Front Immunol. 2022;13:931783.
Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health. 1997;13(4):407–84.
Zhao P. Report from the EMA workshop on qualification and reporting of physiologically based pharmacokinetic (PBPK) modeling and simulation. CPT Pharmacometrics Syst Pharmacol. 2017;6(2):71–2.
Al-Tabakha MM, Alomar MJ. In vitro dissolution and in Silico Modeling shortcuts in bioequivalence testing. Pharmaceutics. 2020;12(1):45.
Rostami-Hodjegan A, Toon S. Physiologically based pharmacokinetics as a component of model-informed drug development: Where we were, where we are, and where we are heading. J Clin Pharmacol. 2020;60(Suppl 1):S12–6.
Wu F, Gao J, Kang J, Wang X, Niu Q, Liu J, et al. Knowledge mapping of exosomes in autoimmune diseases: A bibliometric analysis (2002–2021). Front Immunol. 2022;13:939433.
Williamson B, Riley RJ. Hepatic transporter drug-drug interactions: an evaluation of approaches and methodologies. Expert Opin Drug Metab Toxicol. 2017;13(12):1237–50.
Vijaywargi G, Kollipara S, Ahmed T, Chachad S. Predicting transporter mediated drug-drug interactions via static and dynamic physiologically based pharmacokinetic modeling: A comprehensive insight on where we are now and the way forward. Biopharm Drug Dispos. 2023;44(3):195–220.
Chetty M, Johnson TN, Polak S, Salem F, Doki K, Rostami-Hodjegan A. Physiologically based pharmacokinetic modelling to guide drug delivery in older people. Adv Drug Deliv Rev. 2018;135:85–96.
Kovar L, Schrapel C, Selzer D, Kohl Y, Bals R, Schwab M, et al. Physiologically-Based Pharmacokinetic (PBPK) modeling of buprenorphine in adults, children and preterm neonates. Pharmaceutics. 2020;12(6):578.
Kovar L, Weber A, Zemlin M, Kohl Y, Bals R, Meibohm B, et al. Physiologically-Based Pharmacokinetic (PBPK) modeling providing insights into fentanyl pharmacokinetics in adults and pediatric patients. Pharmaceutics. 2020;12(10):908.
Li Z, Fisher C, Gardner I, Ghosh A, Litchfield J, Maurer TS. Modeling exposure to understand and predict kidney injury. Semin Nephrol. 2019;39(2):176–89.
Guinn D, Sahin L, Fletcher EP, Choi SY, Johnson T, Dinatale M, et al. Pharmacokinetic evaluation in pregnancy-current status and future considerations: Workshop summary. J Clin Pharmacol. 2023;63(Suppl 1):S7–17.
Coppola P, Kerwash E, Cole S. Use of physiologically based pharmacokinetic modeling for hepatically cleared drugs in pregnancy: Regulatory perspective. J Clin Pharmacol. 2023;63(Suppl 1):S62–80.
Wang W, Ye Z, Gao H, Ouyang D. Computational pharmaceutics - A new paradigm of drug delivery. J Control Release. 2021;338:119–36.
Wang W, Ouyang D. Opportunities and challenges of physiologically based pharmacokinetic modeling in drug delivery. Drug Discov Today. 2022;27(8):2100–20.
Lutz JD, Mathias A, German P, Pikora C, Reddy S, Kirby BJ. Physiologically-based pharmacokinetic modeling of remdesivir and Its metabolites to support dose selection for the treatment of pediatric patients with COVID-19. Clin Pharmacol Ther. 2021;109(4):1116–24.
Vieira MLT, Kim MJ, Apparaju S, Sinha V, Zineh I, Huang SM, et al. PBPK model describes the effects of comedication and genetic polymorphism on systemic exposure of drugs that undergo multiple clearance pathways. Clin Pharmacol Ther. 2014;95(5):550–7.
Fairman K, Li M, Ning B, Lumen A. Physiologically based pharmacokinetic (PBPK) modeling of RNAi therapeutics: Opportunities and challenges. Biochem Pharmacol. 2021;189:114468.
Jones HM, Zhang Z, Jasper P, Luo H, Avery LB, King LE, et al. A physiologically-based pharmacokinetic model for the prediction of monoclonal antibody pharmacokinetics from in vitro data. CPT Pharmacometrics Syst Pharmacol. 2019;8(10):738–47.
Kumar M, Kulkarni P, Liu S, Chemuturi N, Shah DK. Nanoparticle biodistribution coefficients: A quantitative approach for understanding the tissue distribution of nanoparticles. Adv Drug Deliv Rev. 2023;194:114708.
D’Mello SR, Cruz CN, Chen ML, Kapoor M, Lee SL, Tyner KM. The evolving landscape of drug products containing nanomaterials in the United States. Nat Nanotechnol. 2017;12(6):523–9.
Acknowledgements
We are grateful to the Department of Pharmacy, the First Affiliated Hospital of Chongqing Medical University and the School of Pharmacy, Chongqing Medical University for supporting this study.
Funding
This work is supported by the Graduate Research Innovation Project of Chongqing, China [grant number CYS23328].
Author information
Authors and Affiliations
Contributions
Xin Wang: Conceptualization, Methodology, Formal analysis, Writing- Original draft preparation. Jiangfan Wu and Hongjiang Ye: Formal analysis, Validation. Xiaofang Zhao: Review and Editing. Shenyin Zhu: Conceptualization, Review and Editing, Supervision, Funding. All the authors have read and approved the final content of this manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Wu, J., Ye, H. et al. Research Landscape of Physiologically Based Pharmacokinetic Model Utilization in Different Fields: A Bibliometric Analysis (1999–2023). Pharm Res 41, 609–622 (2024). https://doi.org/10.1007/s11095-024-03676-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-024-03676-4