Abstract
Purpose
Traditional eye drops exhibit a modest bioavailability ranging from 1 to 5%, necessitating recurrent application. Thus, a contact lens-based drug delivery system presents substantial benefits. Nonetheless, pharmaceutical agents exhibiting poor solubility may compromise the quintessential characteristics of contact lenses and are, consequently, deemed unsuitable for incorporation. To address this issue, the present study has engineered a novel composite drug delivery system that amalgamates micellar technology with contact lenses, designed specifically for the efficacious conveyance of timolol and brinzolamide.
Methods
Utilizing mPEG-PCL as the micellar material, this study crafted mPEG-PCL micelles loaded with brinzolamide and timolol through the film hydration technique. The micelle-loaded contact lens was fabricated employing the casting method; a uniform mixture of HEMA and EGDMA with the mPEG-PCL micelles enshrouding brinzolamide and timolol was synthesized. Following the addition of a photoinitiator, 50 μL of the concoction was deposited into a contact lens mold. Subsequently, the assembly was subjected to polymerization under 365 nm ultraviolet light for 35 min, resulting in the formation of the micelle-loaded contact lenses.
Results
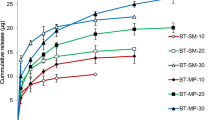
In the present article, we delineate the construction of a micelle-loaded contact lens designed for the administration of brinzolamide and timolol in the treatment of glaucoma. The study characterizes crucial properties of the micelle-loaded contact lenses, such as transmittance and ionic permeability. It was observed that these vital attributes meet the standard requirements for contact lenses. In vitro release studies revealed that timolol and brinzolamide could be gradually liberated over periods of up to 72 and 84 h, respectively. In vivo pharmacodynamic evaluation showed a significant reduction in intraocular pressure and a relative bioavailability of 10.84 times that of commercially available eye drops. In vivo pharmacokinetic evaluation, MRT was significantly increased, and the bioavailability of timolol and brinzolamide was 2.71 and 1.41 times that of eye drops, respectively. Safety assessments, including in vivo irritation, histopathological sections, and protein adsorption studies, were conducted as per established protocols, confirming that the experiments were in compliance with safety standards.
In conclusion
The manuscript delineates the development of a safe and efficacious micelle-loaded contact lens drug delivery system, which presents a novel therapeutic alternative for the management of glaucoma.
Graphical Abstract













Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or its supplementary materials].
References
Patel KD, Silva LB, Park Y. Recent advances in drug delivery systems for glaucoma treatment. Mater Today Nano. 2022;18:100178.
Yanagisawa M, Namekata K, Aida T, Katou S, Tanaka K. EAAT1 variants associated with glaucoma. Biochem Biophys Res Commun. 2020;529(4):943–9.
Mhatre S, Opere CA, Singh S. Unmet needs in glaucoma therapy: the potential role of hydrogen sulfide and its delivery strategies. J Control Release. 2022;347:256–69.
Peral A, Martinez-Aguila A, Pastrana C, Huete-Toral F, Carpena-Torres C, Carracedo G. Contact lenses as drug delivery system for glaucoma: a review. Appl Sci. 2020;10(15):5151.
Carvalho IM, Marques CS, Oliveira RS, Coelho PB, Costa PC, Ferreira DC. Sustained drug release by contact lenses for glaucoma treatment—a review. J Control Release. 2015;202:76–82.
Maulvi FA, Mangukiya MA, Patel PA, Vaidya RJ, Koli AR, Ranch KM, Shah DO. Extended release of ketotifen from silica shell nanoparticle-laden hydrogel contact lenses: in vitro and in vivo evaluation. J Mater Sci Mater Med. 2016;27(6):113.
Alvarez-Lorenzo C, Anguiano-Igea S, Varela-García A, Vivero-Lopez M, Concheiro A. Bioinspired hydrogels for drug-eluting contact lenses. Acta Biomater. 2019;84:49–62.
Xu J, Xue Y, Hu G, Lin T, Gou J, Yin T, He H, Zhang Y, Tang X. A comprehensive review on contact lens for ophthalmic drug delivery. J Control Release. 2018;281:97–118.
Chaudhari P, Ghate VM, Lewis SA. Next-generation contact lenses: towards bioresponsive drug delivery and smart technologies in ocular therapeutics. Eur J Pharm Biopharm. 2021;161:80–99.
Rykowska I, Nowak I, Nowak R. Soft contact lenses as drug delivery systems: a review. Molecules. 2021;26(18):5577.
Sartini F, Menchini M, Posarelli C, Casini G, Figus M. In vivo efficacy of contact lens drug-delivery systems in glaucoma management a systematic review. Appl Sci. 2021;11(2):724.
Rodrigues FS, Campos A, Martins J, Ambrósio AF, Campos EJ. Emerging trends in nanomedicine for improving ocular drug delivery: light-responsive nanoparticles, mesoporous silica nanoparticles, and contact lenses. ACS Biomater Sci Eng. 2020;6(12):6587–97.
Sedlácek J. Possibility of the application of ophthalmic drugs with the use of gel contact lenses [Moznosti aplikace ocních lék gel-kontaktními cockami]. Ceskoslovenská Oftalmologie. 1965;21(6):509–12.
Li CC, Chauhan A. Ocular transport model for ophthalmic delivery of timolol through p-HEMA contact lenses. J Drug Deliv Sci Technol. 2007;17(1):69–79.
Kim J, Chauhan A. Dexamethasone transport and ocular delivery from poly(hydroxyethyl methacrylate) gels. Int J Pharm. 2008;353(1–2):205–22.
Maulvi FA, Desai DT, Shetty KH, Shah DO, Willcox MDP. Advances and challenges in the nanoparticles-laden contact lenses for ocular drug delivery. Int J Pharm. 2021;608: 121090.
Maulvi FA, Choksi HH, Desai AR, Patel AS, Ranch KM, Vyas BA, Shah DO. pH triggered controlled drug delivery from contact lenses: addressing the challenges of drug leaching during sterilization and storage. Colloids Surf B Biointerfaces. 2017;157:72–82.
Maulvi FA, Parmar RJ, Desai AR, Desai DM, Shah DO. Tailored gatifloxacin Pluronic F-68-loaded contact lens: addressing the issue of transmittance and swelling. Int J Pharm. 2020;581(1): 119279.
Maulvi FA, Lakdawala DH, Shaikh AA, Desai AR, Choksi HH, Vaidya RJ, Ranch KM, Koli AR, Vyas BA, Shah DO. In vitro and in vivo evaluation of novel implantation technology in hydrogel contact lenses for controlled drug delivery. J Control Release. 2016;226:47–56.
Kim HJ, Zhang K, Moore L, Ho D. Diamond nanogel-embedded contact lenses mediate lysozyme-dependent therapeutic release. ACS Nano. 2014;8(3):2998–3005.
Maulvi FA, Mangukiya MA, Patel PA, Vaidya RJ, Koli AR, Ranch KM, Shah DO. Extended release of ketotifen from silica shell nanoparticle-laden hydrogel contact lenses: in vitro and in vivo evaluation. J Mater Sci Mater Med. 2016;27(6):1–13.
Wang Z, Li X, Zhang X, Sheng R, Lin Q, Song W, Hao L. Novel contact lenses embedded with drug-loaded zwitterionic nanogels for extended ophthalmic drug delivery. Nanomaterials (Basel). 2021;11(9):2328.
Prisnyi A, Zhilyakova E, Naplekov D, Malyutina A, Bondarev A, Novikov O, Lebedeva O. New drug delivery system in ophthalmology: results studying the surface structure of soft contact lenses from various polymer. BIO Web of Conf. 2021;40:03002.
He H, Lu Y, Qi J, Dong X, Zhao W. In vivo fate of polymeric micelles. In. [C] 0[2024-02-08].
Li Z, Liu M, Ke L, Wang LJ, Wu C, Li C, Li Z, Wu YL. Flexible polymeric nanosized micelles for ophthalmic drug delivery: research progress in the last three years. Nanoscale Adv. 2021;3(18):5240–54.
Naageshwaran V, Bigonne H, Gum G, Malla S, Sol Cd, Bon C, Xu X, Vo A, Smith W, Beringhs AOR, Kozak D, Tan M-L, Babiskin A, Urtti A, Amo EMd, Ranta V-P. Topical pharmacokinetics of brinzolamide suspensions in rabbits and variability analysis for sample size and design considerations. Int J Pharm. 2023;642:123183.
Uner B, Ozdemir S, Pilevne SN, Celebi ARC. Timolol-loaded ethosomes for ophthalmic delivery: reduction of high intraocular pressure in vivo. Int J Pharm. 2023;640: 123021.
Sui Y, Li J, Qu J, Fang T, Zhang H, Zhang J, Wang Z, Xia M, Dai Y, Wang D. Dual-responsive nanovaccine for cytosolic delivery of antigens to boost cellular immune responses and cancer immunotherapy. Asian J Pharm Sci. 2022;17(4):583–95.
Ba-Salem AO, Duhamel J. Determination of the aggregation number of pyrene-labeled gemini surfactant micelles by pyrene fluorescence quenching measurements. Langmuir. 2021;37(19):6069–79.
Jumelle C, Gholizadeh S, Annabi N, Dana R. Advances and limitations of drug delivery systems formulated as eye drops. J Control Release. 2020;321:1–22.
Sun R, Ma S, Chen X, Deng Y, Gou J, Yin T, He H, Wang Y, Tang X, Zhang Y. Inflammation-responsive molecular-gated contact lens for the treatment of corneal neovascularization. J Control Release. 2023;360:818–30.
Peng CC, Kim J, Chauhan A. Extended delivery of hydrophilic drugs from silicone-hydrogel contact lenses containing Vitamin E diffusion barriers. Biomaterials. 2010;31(14):4032–47.
Nguyen DCT, Dowling J, Ryan R, McLoughlin P, Fitzhenry L. Pharmaceutical-loaded contact lenses as an ocular drug delivery system: a review of critical lens characterization methodologies with reference to ISO standards. Cont Lens Anterior Eye. 2021;44:101487.
Mehta P, Al-Kinani AA, Arshad MS, Singh N, Ahmad Z. Engineering and development of chitosan-based nanocoatings for ocular contact lenses. J Pharm Sci. 2018;108(4):1540–51.
Fernandes AC, Silva D, Nunes TG, Colaco R. The effect of albumin and cholesterol on the biotribological behavior of hydrogels for contact lenses. Acta Biomater. 2015;26:184–94.
Maulvi FA, Kanani PA, Jadav HJ, Desai BV, Desai DT, Patel HP, Shetty KH, Shah DO, Willcox MDP. Timolol-eluting graphene oxide laden silicone contact lens: control release profile with improved critical lens properties - ScienceDirect. J Drug Deliv Sci Technol. 2022;69:103134.
Maulvi FA, Soni TG, Shah DO. Extended release of timolol from ethyl cellulose microparticles laden hydrogel contact lenses. Open Pharm Sci J. 2015;2(1):1–12.
Xu J, Ge Y, Bu R, Zhang A, Feng S, Wang J, Gou J, Yin T, He H, Zhang Y, Tang X. Co-delivery of latanoprost and timolol from micelles-laden contact lenses for the treatment of glaucoma. J Control Release. 2019;305:18–28.
Draize JH, Woodard G, Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 1944;82(3):105–7.
Li Z, Liu M, Ke L, Wang LJ, Wu C, Li C, Li Z, Wu YL. Flexible polymeric nanosized micelles for ophthalmic drug delivery: research progress in the last three years. Nanoscale Adv. 2021;3(18):5240–54.
Fuentes R, Fernández E, Pascual I, et al. UV-visible transmittance of silicone-hydrogel contact lenses measured with a fiber optic spectrometer[C] Iberoamerican optics meeting & latin american meeting on optics. Int Soc Opt Photonics. 2013. https://doi.org/10.1117/12.2025710.
Efron N. Development of contact lenses from a biomaterial point of view – materials, manufacture, and clinical application[J]. Compr Biomater. 2011:517–541. https://doi.org/10.1016/B978-0-08-055294-1.00270-1.
Yang H, Zhao M, Xing D, Zhang J, Fang T, Zhang F, Nie Z, Liu Y, Yang L, Li J, Wang D. Contact lens as an emerging platform for ophthalmic drug delivery: a systematic review. Asian J Pharm Sci. 2023;18(5):100847.
Insua Pereira E, Lira M. Comfort, ocular dryness, and equilibrium water content changes of daily disposable contact lenses. Eye Contact Lens. 2018;44 Suppl 2:S233–240. https://doi.org/10.1097/ICL.0000000000000441.
Tranoudis I, Efron N. Parameter stability of soft contact lenses made from different materials. Cont Lens Anterior Eye. 2004;27(3):115–31.
Nasr FH, Khoee S, Dehghan MM, Chaleshtori SS, Shafiee A. Preparation and evaluation of contact lenses embedded with polycaprolactone-based nanoparticles for ocular drug delivery. Biomacromolecules. 2016;17(2):485–95. https://doi.org/10.1021/acs.biomac.5b01387.
Lin MC, Svitova TF. Contact lenses wettability in vitro: effect of surface-active ingredients. Optom Vis Sci. 2010;87(6):440–7.
Campbell D, Carnell SM, Eden RJ. Applicability of contact angle techniques used in the analysis of contact lenses, part 1: comparative methodologies. Eye Contact Lens. 2013;39:254–62.
Acknowledgements
This work was supported by the Scientific Research Project of Liaoning Province Education Department (2020LJC16).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors have declared no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic srrupplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, H., Zhang, F., Fan, Y. et al. Co-delivery of Brinzolamide and Timolol from Micelles-laden Contact Lenses: In vitro and In Vivo Evaluation. Pharm Res 41, 531–546 (2024). https://doi.org/10.1007/s11095-024-03672-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-024-03672-8