Abstract
Purpose
Intratracheal delivery and consistent dosing of dry powder vaccines is especially challenging in mice. To address this issue, device design of positive pressure dosators and actuation parameters were assessed for their impacts on powder flowability and in vivo dry powder delivery.
Methods
A chamber-loading dosator assembled with stainless-steel, polypropylene or polytetrafluoroethylene needle-tips was used to determine optimal actuation parameters. Powder loading methods including tamp-loading, chamber-loading and pipette tip-loading were compared to assess performance of the dosator delivery device in mice.
Results
Available dose was highest (45%) with a stainless-steel tip loaded with an optimal mass and syringe air volume, primarily due to the ability of this configuration to dissipate static charge. However, this tip encouraged more agglomeration along its flow path in the presence of humidity and was too rigid for intubation of mice compared to a more flexible polypropylene tip. Using optimized actuation parameters, the polypropylene pipette tip-loading dosator achieved an acceptable in vivo emitted dose of 50% in mice. After administering two doses of a spray dried adenovirus encapsulated in mannitol-dextran, high bioactivity was observed in excised mouse lung tissue three days post-infection.
Conclusions
This proof-of-concept study demonstrates for the first time that intratracheal delivery of a thermally stable, viral-vectored dry powder can achieve equivalent bioactivity to the same powder, reconstituted and delivered intratracheally. This work may guide the design and device selection process for murine intratracheal delivery of dry powder vaccines to help progress this promising area of inhalable therapeutics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For the prevention of respiratory infectious diseases like tuberculosis, dry powder vaccine delivery via a pulmonary route is very promising as the lungs are highly vascularized and offer a large surface area for absorption [1,2,3]. By directly targeting the lungs, highly localized therapeutic doses can be delivered with reduced systemic side effects [4] and may even require a lower therapeutic dosage to initiate a mucosal immune response compared to systemic intramuscular delivery [5]. Biologics encapsulated in dry powder form have the additional advantage of displaying thermal stability at elevated temperatures that minimizes the need for expensive cold-chain infrastructure to maintain vaccine efficacy [6]. The non-invasive nature of inhaled delivery and relative ease of self-administration makes inhalation highly desirable for dry powder vaccine administration.
In early stages of vaccine development, preclinical animal models are critical for determining the immunogenicity and reactogenicity profiles of a new therapeutic [7]. Murine models are of particular importance in the preclinical screening of vaccines, serving as a relatively inexpensive mechanistic model with a short growth period [3, 8, 9]. However, testing inhalable dry powder vaccine products with murine models is especially challenging since active inhalation cannot be directly controlled and the small anatomical scale of mice can lead to inconsistencies in dosage delivery. Pulmonary delivery in a murine model is further complicated due to the lack of commercially available devices that can be used for consistent dosing. As a result, groups studying dry powder administration to mice have resorted to developing custom-made dosage chambers or hand-held devices with varying levels of complexity [2, 7, 10, 11].
Current pulmonary delivery strategies used for rodents range from passive inhalation to intratracheal and endotracheal administration, often referred to as insufflation. Since mice are obligatory nose-breathers, passive inhalation requires aerosolized powder to travel through the nasal cavity prior to reaching the trachea and primary bifurcation of the lungs [12]. This approach typically utilizes a customized chamber set-up to ensure uniform powder dispersion in the enclosed space, commonly suffering from poor delivery efficiency and very high powder mass requirements [2, 3, 10]. For high-cost biologics like viral-vectored vaccines that are produced in small-batch quantities, passive powder inhalation in mice is not usually a feasible approach for delivery.
A small range of handheld insufflation devices have been developed for intratracheal dry powder delivery in mice using positive pressure from a syringe to dispel powder [7, 11, 13, 14]. Similar to the Penn-Century Dry Powder Insufflator™, which is no longer commercially available, these intratracheal dosator devices are loaded with a small mass of dry powder that is sprayed through a needle tip directly into the trachea. Depending on the device design, initial powder loading has been demonstrated using a tamping strategy [14], chamber-loading method [11], or direct loading into a pipette needle tip [7, 13]. Although these devices can be assembled with easily accessible components, there is a lack of general guidance towards material selection and resulting compatibility with a dry powder formulation. To the best of our knowledge, the impact of dosator device design and corresponding powder loading method has never been directly quantified or compared for a highly compressible dry powder vaccine.
To optimize intratracheal delivery using a custom-made dosator device, this study evaluates how design and operational parameters impact device performance. Material selection of the dosator tip, selected actuation parameters and powder loading strategy are all expected to impact the performance of custom-made intratracheal dosators in terms of achieving consistent and sufficient emitted dose into mice. By testing a chamber-loading dosator design with a stainless steel, polypropylene, or polytetrafluoroethylene (PTFE) needle tip, the impact of operational parameters like loaded powder mass, air volume, and device storage conditions on powder flowability were evaluated. Assembly of a chamber-loading dosator offered the highest level of customization in terms of needle tip material and powder capacity. As such, the chamber-loading design was used as the basis for investigating the impact of actuation parameters, with the expectation that these findings would be transferrable to other applicable dosator designs.
Upon determining the optimal actuation parameters, we compared three different dosator loading methods which use tamp-loading, chamber-loading, and pipette-tip loading to assess the in vivo intratracheal device functionality and resulting bioactivity of a thermally stable spray dried powder containing adenovirus. Intended as a viral-vectored vaccine, this human serotype 5 adenovirus (AdHu5) encapsulated in a binary excipient mixture of mannitol and dextran at a 1:3 ratio by mass has previously been shown to retain viral activity after 72-h exposure to 45 ℃ with only 0.8 log loss in viral titre [15]. Testing in mice supported the optimization of the dosator device design and indicated that sufficient bioactivity can be achieved for future vaccine studies. Overall, this study is intended to provide guidance on dosator selection for validating formulations of inhalable dry powder viral biopharmaceutics with murine models.
Materials & Methods
Chemicals and Biologics
D-mannitol at USP grade and D-(+)-trehalose dihydrate were purchased from Millipore-Sigma (ON, Canada) while dextran (Mr 500000 Da) was purchased from ThermoFisher Scientific (Waltham, MA, USA). A Barnstead GenPure Pro system (ThermoFisher Scientific, Waltham, MA, USA) was used to purify Milli-Q® water at a resistivity of 18.2 MΩ-cm. Preparation and purification of a recombinant, replication deficient human adenovirus of serotype 5 expressing Luciferase (AdHu5-Luc) was conducted at the vector facility within the McMaster University Immunology Research Centre. The adenoviral vector stock was suspended in a 5% trehalose storage buffer directly after purification with a titer of \(3.5 \times {10}^{9} \mathrm{PFU}/\mathrm{mL}\) and was stored at -80 ℃ based on a previously optimized protocol [6]. Luciferase assay substrate, luciferase assay buffer and 5X cell culture lysis reagent were all purchased from Promega (Madison, WI, USA) as part of a luciferase assay system.
Spray Dried Powder Preparation
A mannitol-dextran excipient solution was prepared by dissolving mannitol and dextran at a 1:3 mass ratio in purified Milli-Q® water to a final concentration of 1% solids. This formulation was selected based on our previous study that minimized viral log loss during spray drying and thermal aging [15]. For all testing described in the Dosator Performance Testing Section, a 5% trehalose placebo solution was added to the excipient blend at 60 µL/100 mg excipient, while in vivo testing required the addition of the AdHu5-Luc stock vector at the same concentration of 60 µL/100 mg excipient. A previous study has shown that mannitol-dextran spray dried powders have constant particle size and morphology with and without the addition of AdHu5 [15], so placebo testing is expected to accurately represent the powder flow of the adenoviral powder.
Once dissolved, the excipient solution was spray dried through a 0.7 mm nozzle using a B-290 Mini Spray Dryer (Büchi, Switzerland). The following processing conditions were selected based on previous optimization to maximize yield and viral activity while achieving inhalable particle size [15]: feed flow rate of 217.5 mL/h, spray gas flow rate of 439.11 L/min, aspirator flow rate of 35 m3/h and an inlet temperature of 120 ℃. An outlet temperature range between 55–65 ℃ was recorded. Powder was collected immediately after drying and transferred to microcentrifuge tubes within a biosafety cabinet. A benchtop desiccator filled with Drierite® anhydrous indicating desiccant (W.A Hammond Drierite Company Ltd.) was used for short-term room temperature storage to avoid ambient moisture uptake.
As an indicator of spray drying efficiency, we have previously reported a collected yield of 13 \(\pm\) 2% for this viral powder formulation when spray drying a low batch feedstock volume of 10 mL [6]. It should be noted that collected yield represents the amount of powder removed from the glass collection vial of the spray dryer and is an underrepresentation of the total yield produced. For the purposes of this study, larger batch sizes were prepared to improve collected yield and overall spray drying efficiency.
Intratracheal Dosator Assembly
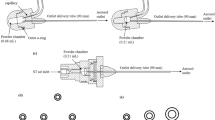
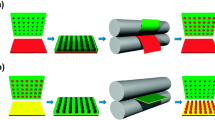
Three custom-made dosator designs were assembled and primarily differed based on the method of powder loading (Fig. 1). The tamp-loading design was based on a loading method described by Stewart et al. [14] and was fabricated using a 22 Ga blunt tip intravenous catheter with 1.5 inch stainless steel needle tip and a 1 mL syringe press-fit at the opposite end as shown in Fig. 1A. This device required minimal assembly using two prefabricated components, including the intravenous catheter and syringe. On average, the tracheal diameter of a mouse is approximately 1.5 mm [16], which makes a 22 Ga or 23 Ga catheter needle an appropriate size for intratracheal delivery. Powder loading was accomplished using a previously described method in which the needle tip was repeatedly tamped in a small plastic tamp bucket containing approximately 20 mg of powder [14]. The dosator was weighed before and after tamping to determine the loaded powder mass.
Assembled dosator designs intended for intratracheal dry powder delivery which differ based on the loading strategy, including: (A) tamp-loading (B) chamber-loading and (C) pipette-tip loading. Three tip materials (D) were tested for the chamber-loading design including stainless steel, polypropylene and PTFE. Design performance was tested using a vertical experimental set-up directing powder flow through a 3D printed mouse trachea and into a collection vial (E).
Comparatively, the chamber-loading design (Fig. 1B) was based directly on the described protocol by Durham et al. [11] of a disposable dosator for pulmonary insufflation in small animals. The powder loading chamber was prepared using a 0.5 mL microcentrifuge tubes with holes drilled in the center of the cap and bottom of the tube with diameters of approximately 4 mm and 2 mm, respectively. Blunt end 1.5 inch, luer lock-connecting 23 Ga needle tips made from stainless-steel (Amazon, Seattle, WA, USA), polypropylene (McMaster-Carr, Elmhurst, IL, USA) and PTFE (McMaster-Carr, Elmhurst, IL, USA) were used to compare the effect of tip material on powder flow from a chamber-loading dosator design (see Fig. 1D). The needle tip was press-fit directly into the bottom of the microcentrifuge tube and with the cap open, powder was carefully loaded into the microcentrifuge chamber. A small piece of cotton was placed near the top of loading chamber before closing the cap to prevent backflow of material upon actuation of the syringe secured in the top hole of the microcentrifuge cap. Unlike the design by Durham et al. [11], a fine mesh screen between the loading chamber and needle tip was not used in this design, based on initial testing that showed poor powder flow from the device (data not shown).
Finally, the pipette tip-loading dosator design used a 3-way stopcock from Cole-Parmer (Montreal, Canada) with a luer inlet for connecting a syringe with a complementary male luer lock, adapted from similar designs reported by Ihara et al. and Qui et al. [7, 13]. In this design, the 3-way stopcock served as a connection between the prefabricated pipette tip and the 1 mL syringe. As previously described, our version of the pipette tip-loading dosator used a 10 µL Rainin LiteTouch System polypropylene pipette tip with surface repellent (Mettler-Toledo, Oakland, CA, USA) in which powder was carefully poured using weigh paper prior to securing on the opposite end of the three-way stopcock, as indicated in Fig. 1C [15]. All three components of this dosator design, including the pipette tip, 3-way stopcock and syringe were prefabricated and required minimal assembly.
Dosator Performance Testing
To determine the impact of various operational parameters on dosator functionality, a range of syringe air volumes, loaded masses, storage times and storage conditions were evaluated. Storage in this case referred to the inactive period between loading the dosator in the lab and dose delivery to mice within the animal facility on campus. Syringe air volumes ranged from 0.3 to 0.9 mL, based on reported values that have been previously used for successful insufflation in mice [7, 17]. The total lung capacity of a mouse is approximately 1 mL, which served as the critical upper limit of aspiration volumes used for dosator actuation [17]. Dosator performance was assessed in terms of powder flowability from the device and quantified based on available dose (defined below). As shown in Fig. 1E, a testing set-up was used to vertically spray a loaded dosator through a 3D printed mouse trachea (created from a CT scan of a healthy mouse) and into a glass collection vial placed directly below, using a method previously described [15]. Each 23 Ga needle tip fit snuggly within the inner diameter of the 3D printed trachea. A retort stand with clamp was used to ensure the loaded dosator assembly was sprayed from a consistent height and properly centered above the collection vial. All tests with the 3D printed trachea were conducted with the chamber-loading dosator design, based on the ability to load a wider range of powder mass and ease in testing different needle tip materials. Available dose was used to describe the percentage of placebo powder exiting the dosator with respect to the total amount of powder initially loaded, as indicated in Eq. 1. Each sample condition was measured in triplicate and error bars represent standard error between repeated measurements.
The expected powder dose delivered to the mouse lung tissue was approximated by the mass of powder collected within the glass vial. Here, we refer to the delivered dose as the percentage of powder that can flow through the 3D printed trachea compared to the total powder initially loaded, as provided by Eq. 2. Differences in the average available dose and average delivered dose between sample conditions were considered to be statistically significant when overlap between error bars was not observed.
Intratracheal Inoculation for Liquid Delivery In Vivo
Female BALB/c mice 8–10 weeks old were purchased from Charles River (Wilmington, USA) and housed in a level B facility at McMaster University for one week prior to all in vivo experiments. All experimental procedures and animal handling protocols were approved and in accordance with the guidelines of McMaster University’s Animal Research and Ethics Board. To confirm suitability of our excipient blend and quantify in vivo bioactivity of the adenovirus following spray drying, reconstituted spray dried powder was administered as a liquid control via intratracheal inoculation using a previously described technique [18]. Following anaesthetization with isoflurane, mice were hung by their teeth on a string attached to a 45° intubation board while the tongue is held to the side to expose the trachea and prevent swallowing. A pipette was used to deliver liquid drops of reconstituted spray dried powder to the back of the mouth, allowing each drop to be individually aspirated inhaled. Two doses were administered within 1 h, with each dose consisting of 2.4 mg of spray dried powder reconstituted in 40 µL PBS. The total viral dosage per animal was \(2\times {10}^{6}\) PFU. Reconstituted viral powder was delivered to three animals to assess statistical variability.
Intratracheal Powder Delivery In Vivo
For in vivo testing of dry powder, intratracheal dosators were loaded and stored in a plastic bag that was sealed within a glass container filled with desiccant for transport to the animal facility. For all tests conducted under ambient humidity above 10% RH, the glass container was additionally placed on dry ice during storage and transport prior to administration in animals. Spray dried powder was not directly in contact with the dry ice, as the glass container prevented exposure to CO2 sublimation which is known to cause acidification that can deactivate adenovirus [19].
To deliver spray dried powder using each dosator design, endotracheal intubation was conducted using a previously described protocol in which the anesthetized mouse was suspended on a 45° intubation board by its teeth [18]. Once the tracheal opening was located, a P200 pipette containing 100 µL of water was inserted into the trachea to confirm the correct location based on the movement of the air gap during aspiration. The P200 pipette tip was then removed and replaced with the needle tip of the loaded dosator for subsequent actuation. Each dosator was loaded with powder and weighed, prior to device administration in animals and directly afterwards. The in vivo performance of each dosator design was determined based on the change in dosator weight before and after administration and was used to calculate emitted dose. All powder was delivered using a syringe air volume of 0.6 mL through fast actuation. Mice that did not receive any reconstituted or dry powder treatment were considered the negative control group. A spray dried placebo powder containing no added vector was also tested for baseline comparison. As with the reconstituted powder delivery, two spray dried powder doses of approximately 1–4 mg each were sprayed within a 1-h interval for a total viral dosage between \(2\times {10}^{6}\) and \(3\times {10}^{6}\) PFU. Animals were sacrificed 3 days post-infection and lung tissue was removed for storage at -70 ℃. Three animals were used to assess each treatment group, including the negative control, placebo powder and spray dried viral powder.
In Vivo Adenovirus Bioactivity via Luciferase Assay
Luciferase activity within collected lung tissue was assessed after approximately 24 h of cryo-storage. Frozen lung tissue was transferred to 14 mL polypropylene tubes containing 1 mL of a 1X solution of cell culture lysis reagent, stored on ice. Samples were fully homogenized with a Brinkmann Homogenizer (Polytron, Switzerland) on ice for 30–40 s before centrifugation at 3000 rpm for 10 min at 4 ℃. Supernatants were transferred into 1 mL microcentrifuges tube on ice, while the luciferase assay reagent was thawed to room temperature. In a Corning® Costar 96-well white solid plate (Thermo Fisher Scientific, Waltham, USA), 100 µL of luciferase assay reagent mixed with 20 µL of sample supernatant per well, followed by gentle pipette mixing. Luminescence was immediately measured using a i3SpectraMax Plate Reader (Molecular Devices, CA, USA). Three wells were analyzed per sample and the average signal was used to determine the relative luciferase units within the total lung tissue, which accounted for the sample dilution prior to homogenization. In each treatment group, the average luciferase activity and standard error was determined using lung tissue collected from a sample size of three animals. Average luciferase activity of each treatment group was compared using standard error, where overlap in the error bars indicated no statistically significant difference between treatment groups.
Statistical Analysis
The statistical variability of all dosator performance measurements and in vivo bioactivity quantification were analysed using Microsoft Excel. Standard error of the mean was used to assess the relative variation between treatment groups and the random variation within each sample group. To assess statistical differences observed between dosator performance parameters, as well as difference between in vivo treatment groups, two sample t-tests were conducted using Microsoft excel, whereby p-values < 0.05 indicated that the difference between compared averages was statistically significant.
Results and Discussion
Impacts of Air Volume on Chamber-Loading Dosator
Achieving incipient flow through the needle tip with our cohesive mannitol-dextran powder required sufficient air pressure to overcome interparticle forces and wall friction to effectively aerosolize the powder from its bulk stored state. To investigate the impact of syringe air volume on device performance, chamber-loading dosators were assembled with three needle tips of differing materials, loaded with placebo powder and sprayed using 0.3, 0.6 and 0.9 mL of air preloaded in a syringe (Fig. 2). For both stainless-steel tip and PTFE tip, device performance was poor for the two lower air volumes but substantially improved to approximately 20% available dose when a 0.9 mL syringe volume was used. This difference in dosator performance when using 0.3 mL syringe volume compared to 0.9 mL was statistically significant, as indicated by the calculated p-values of 0.023 and 0.013 for the stainless steel and PTFE tips, respectively. With progressively greater volumes of air, the solids were more effectively dispersed from the dosator needle tip. Comparatively, the polypropylene tip had marginally better performance at the two lower air volumes though it did not improve with 0.9 mL air volume like the other cases. Despite increased air volume, consistent dispensing of the bulk solids with the polypropylene tip was likely due to the tendency of this powder formulation to build up static charge during spray drying [20] which is held in balance by the dielectric properties of polypropylene [21]. Charge stabilization by PTFE was less evident than it was for polypropylene, but the significance of static charge and the resulting impact on powder flow from the dosator should not be overlooked.
Available dose and estimated delivered dose collected from a chamber-loading dosator design assembled with a (A) stainless steel tip, (B) polypropylene tip and (C) PTFE tip. Powder was administered from the dosator device using syringe air volumes of 0.3 mL, 0.6 mL and 0.9 mL. Statistically significant differences (p-value < 0.05) in dosator performance are indicated (*).
For all tip materials, the delivered dose was similar to the available dose, indicating effective powder flow through the 3D printed trachea. Minimal powder losses on the 3D printed tracheal walls were observed by eye which suggested the powder formulation and delivery strategy were suitable for inhalation in mice. Maximizing available dose, and similarly improving delivered dose, was paramount for achieving overall delivery efficiency and reducing powder wastage within the device itself. Despite the general improvements in powder emission with 0.9 mL air volume, this volume is too close to the critical limit for safe delivery to a mouse without risk of tissue damage. Qiu et al. [7] found that actuation of an intratracheal dosator using 1 mL of air caused weight change in BALB/c mice between 7–9 weeks old, suggesting that tissue damage should be anticipated at or beyond an air volume of 0.9 mL. As a result, 0.6 mL was elected as the optimum air volume for devices in this study, with new focus placed on other actuation parameters to maximize delivery efficiency.
Loaded Mass Optimization of Chamber-Loading Dosator
The optimum loaded mass for the chamber-loading dosator was determined by examining a range of masses for each of the three needle tip materials, with the results summarized in Fig. 3 for 0.6 mL dispensed air volume. Care was taken to minimize vibrations to the dosator during these tests to avoid accelerating the time consolidation of loaded powders. Despite all loading chambers being the same size, with similar needle tip geometry, there were distinct differences in dosator performance with different initial powder mass loading. When a stainless-steel tip was used, available dose decreased significantly (p-value = 0.009) after the loaded mass exceeded 7 mg (Fig. 3A). Above 7 mg, significant powder agglomeration was occurring at the entrance to the needle tip, causing an intermittent blockage during use. Within the optimum loading range below 7 mg, the stainless-steel tip outperformed the other tip materials in terms of available dose. Since these powders contained very low moisture content, <3 wt.% [15], static build-up was more effectively dissipated by the stainless-steel tip, compared to the other materials, allowing for more effective flow from the chamber-loading device.
Available dose and estimated delivered dose (sprayed immediately after loading) collected from an intratracheal dosator with chamber-loading design. Powder dose was normalized based on initial powder mass loaded into the chamber of a dosator device assembled with (A) stainless steel tip, (B) polypropylene tip and (C) PTFE tip and sprayed using 0.6 mL air volume. Statistically significant differences (p-value < 0.05) in dosator performance are indicated (*) for each respective dosator tip material.
Comparatively, the polypropylene tipped dosator had a relatively constant available dose up to 11 mg, similar to the trend seen for air volume, which suggests that it would be more effective when a high powder dosage was required to elicit an immunogenic response (Fig. 3B). The PTFE tip performed poorly across the tested mass range, but available dose improved slightly at the 10–11 mg loading (Fig. 3C) to indicate a statistically increase significant in dosator performance (p-value = 0.018) compared to lower mass loading. Significant build-up of powder across the inner length of the needle tip indicated that static attraction occurred between the PTFE needle tip and the dry mannitol-dextran powder. With a higher mass loading, the higher applied stresses from the syringe on the powder were sufficient to dilate segments of the bulk solids in the PFTE needle tip to allow for better particle travel and powder emission.
As shown in Fig. 2, there was no statistically significant difference between delivered dose and available dose, indicating appropriate powder flow of this formulation through the model trachea. Although the available dose percentage should be ideally maximized, the optimal mass of a virus-containing dry powder that is delivered per dosator actuation will be highly dependent on the viral dosage required to elicit an immune response, as well as the viral titre of the dry powder [6]. Conversely, due to the anatomically small scale of a mouse’s lungs, too much powder delivered in a single spray can pose a choking hazard. Other studies have suggested that 1–2 mg of powder is appropriate for intratracheal delivery [7, 17], but our initial tests indicated that up to 6 mg can be safely delivered intratracheally (data not shown). The delivery efficiency of the stainless-steel tip dosator reached approximately 45% available dose when 6–7 mg of powder was loaded, which meant that approximately 3 mg of the powder could be delivered. For the polypropylene tip that achieved a delivered dose of 25% when loaded with 10–11 mg of powder, approximately 2.6 mg delivered powder was considered an acceptable powder dosage. On the other hand, the PFTE tip dosator did not dispense a suitable powder mass, with only 8% available dose at 10–11 mg loading, which meant that approximately 0.8 mg of powder was delivered. The PTFE needle tip had higher surface roughness compared to stainless-steel or polypropylene, leading to an increased internal surface area within the needle tip. As a result, it was speculated that the PTFE needle-tip had more direct contact with the powder which hindered powder flow.
Recognizing the recommended upper limit of powder mass that can safely be delivered to a mouse per dose, the loaded mass must be considered in conjunction with spray efficiency to successfully deliver an appropriate powder dosage. Preliminary in vivo testing in mice using luciferase expression as a marker of adenovirus infection revealed that a minimum liquid dosage of \(1\times {10}^{6} \mathrm{PFU}\) delivered intratracheally was required to produce a bioactive response in mice (via luciferase assay described in the Methods section, data not shown). For the viral-vectored dry powder used in this study, the viral titre of the dry powder after spray drying was \(4.2\times {10}^{5} \mathrm{PFU}/\mathrm{mg}\), therefore a target powder dosage of at least 2.3 mg was required for quantifiable viral infection and gene expression. Since the PTFE tip could not deliver this mass of mannitol-dextran powder with the loaded mass range tested, it was not suitable for our intended in vivo application. Comparatively, chamber-loading dosators outfitted with stainless steel and polypropylene needle tips could both emit at least 2.3 mg, when loaded with the optimal mass of 6–7 mg and 10–11 mg, respectively. However, the stainless-steel tip was preferred between these two alternatives since it offered a higher available dose with lower mass loaded, resulting in higher device efficiency and reduced powder wastage.
Impacts of Dosator Storage Conditions
Since the time between loading a dosator and spraying in vivo can vary substantially, particularly when a prepared dose must be transported to an animal testing facility, the impact of ambient storage time and consolidation was necessary to assess device efficiency (Fig. 4). Recall that our practice for in vivo testing (discussed above) stored the dosator on dry ice to protect from moisture, but here we first present results with no dry ice present to quantify the effects of room humidity on dosator performance. At the time of testing, ambient humidity was ~40% RH which was observed to impact powder behaviour and dosator performance. The results in Fig. 4A show a notable decline in the available and delivered dose for the stainless-steel tip design over time in this humidity. There was a statistically significant difference in the available dose (p-value = 0.027) between 15 and 30 min of ambient storage time compared to available dose measured after 60 and 90 min of ambient storage. Prolonged storage under ambient conditions led to visible powder clumping within the loading chamber of the stainless-steel dosator which limited the amount of powder that could flow from the needle tip. Conversely, the device with the polypropylene tip did not show the same decline in performance over time under ambient conditions, but there was notably high variability and poor repeatability between sprays (Fig. 4B). There was a statistically significant increase in available dose (p-value = 0.009) from 15 to 30 min of ambient storage. The hydrophobic nature of polypropylene may provide a beneficial water repellent effect [22], preventing water adsorption onto the interior surface of the needle tip. Once the powder was in motion, this repellent effect prevented the powder from contacting any additional water source which would have otherwise promoted further agglomeration. The mannitol-dextran powder used in this study is hydrophilic due to its carbohydrate nature, which contributes to its moisture sensitivity and promotion of agglomeration in the stainless-steel tip. But when there was no attraction to water by the tip itself, this leads to a repulsion effect between particle and tip wall. In comparison, the PTFE tip showed poor available dose performance overall and notably lower available and delivered dose at the 90-min timepoint (Fig. 4C). Although PTFE is highly hydrophobic, like polypropylene, the internal luer lock geometry connecting the PTFE needle and the loading chamber seemed to induce powder consolidation that limited particle flow.
Performance of chamber loading dosators after loading powder and exposing the assembly to ambient conditions (A–C) or dry ice (D–F) for designated storage times prior to administration. Available dose and estimated delivered dose were normalized based on the amount of initial powder loaded, which was optimized based on tip material selection according to Fig. 3. Statistically significant differences in performance (p-value < 0.05) between initial and prolonged ambient storage times and between ambient and dry ice storage conditions are denoted as (*).
An alternative storage protocol was considered to address device performance concerns under high humidity conditions by storing the pre-loaded dosators within a glass container surrounded by dry ice for various time periods prior to actuation. When using dry ice storage, significant improvements in both available and delivered dose were observed for all tip materials, specifically at longer storage times (Fig. 4D, E, F). After 90 min of storage time on dry ice, the stainless-steel design yielded an available dose of 10% compared to 2% available dose when stored at ambient conditions for the same time interval. With a p-value of 0.038, the difference is statistically significant between available dosage at 60 min and 90 min of storage on dry ice compared to storage at ambient conditions. After 30 min of storage, the average available dose from the polypropylene tip showed a slight increase from 37 to 43% (p-value = 0.034) with the use of dry ice and a significant reduction in variability between measurements at most tested time points (indicated by smaller error bars in Fig. 4E). The use of dry ice storage also improved the average available dose observed at the 90-min timepoint for the PTFE tip design, increasing to 12% compared to 3% under ambient conditions. The calculated p-value of 0.094 indicated significant difference between dosator performance under ambient conditions compared to dry ice at 60 and 90 min of storage time. This improvement in dosator performance was due to the cooling effect created by dry ice sublimation which caused water vapour in the surrounding air within the glass container to condense [23]. As a result, ambient humidity decreases to prevent moisture uptake and agglomeration of the spray dried powder. Overall, careful dry ice storage was preferrable for improved dosing consistency and general performance of a chamber-loading dosator when working in environments with elevated humidity.
In Vivo Dosator Design Comparison
Using the optimal actuation parameters, device storage conditions and tip materials detailed above, in vivo performance of three dosator loading methods were evaluated. A stainless-steel tip was selected for both the tamp-loading dosator and the chamber-loading dosator as this tip material required less loaded powder mass to achieve a sufficient available dosage during testing (Fig. 3). The rigidity of the stainless-steel needle also improves accuracy when targeting powder delivery to the trachea. Although polypropylene needle-tips led to high available dose (Fig. 4), these needle tips were not selected for tamp-loading and chamber-loading dosator designs due to their flexible nature and difficultly in physically aligning with the trachea during initial mouse testing. However, performance of a pipette-tip dosator loading method was directly compared using a rigid, commercially available polypropylene tip offering beneficial hydrophobic properties. An air volume of 0.6 mL, optimal loaded mass and dry ice storage was used for all experimentation that occurred with ambient humidity exceeding 10% RH to analyze the three dosator loading methods. Based on initial in vivo testing (data not shown), dosator actuation after storage in a humid environment without dry ice resulted in insufficient emitted dose and an undetectable bioactive response in mice.
Using spray dried powder containing AdHu5-Luc vector at a viral potency of \(4.2\times {10}^{5} \mathrm{PFU}/\mathrm{mg}\), the emitted dose of each dosator design is presented in Table I. Overall, the tamp-loading design with stainless-steel tip offered low powder loading capacity and could not successfully emit powder in vivo to achieve a measurable powder dosage. Due to the dense consolidation experienced during tamp-loading, the loaded powder solidified in the tip and could not be successfully sprayed intratracheally. In addition, the proximity of the powder to the end of the stainless-steel tip led to moisture absorption and subsequent agglomeration when the tip came in contact with the humid environment of the oropharynx, preventing all powder flow from the dosator tip. Comparatively, the chamber-loading design had a slightly higher mass loading capacity with an in vivo emitted dose of 15% that resulted in 0.9 mg of powder sprayed (Table I). However, this emitted dose was still too low to achieve sufficient viral potency for an observable bioactive response. Fortunately, the pipette tip-loading design provided a much higher emitted dose in vivo, allowing for sufficient viral dose delivery and assessment of adenovirus bioactivity. By choosing a low retention pipette tip made of polypropylene, the surface coating likely increased the hydrophobicity of the interior surface which enhanced the positive performance of this material described in previous sections. The increased hydrophobicity created a water-repellent effect that helped to limit powder adhesion and encourage particle flow. When using a pipette tip-loading strategy, Qiu et al. [7] have similarly reported strong device performance and indicated that there was negligible moisture absorption at the delivery site in the oropharynx with this method.
Using the pipette tip-loading dosator, bioactivity of the spray dried AdHu5-Luc vector was assessed in vivo along with an uninfected negative control and spray dried placebo excipient blend as well as reconstituted powder delivered as a liquid (Fig. 5). It should be noted that luciferase is used as a marker to indicate cell infection and subsequent gene expression, testing the immunogenicity of the intended viral-vectored vaccine antigen will require additional study. In this experiment, two powder doses were delivered to achieve a total viral dosage of \(2\times {10}^{6} \mathrm{PFU}\), ensuring a strong and repeatable luciferase signal could be easily quantified. Compared to the uninfected and placebo powder controls, mice that received either reconstituted spray dried powder or spray dried powder via the pipette tip-loading dosator both showed very strong luciferase expression with a statistically significant difference between the non-viral treatments (p-value = 0.013). This indicated that the adenoviral vector successfully infected the targeted lung tissue and to the best of our knowledge, this is the first report which demonstrates that a thermally stable, spray dried AdHu5 vector can be effectively delivered intratracheally in mice to yield a strong bioactive response. As expected, there was no statistically significant difference between the bioactivity observed in uninfected mice and mice which received placebo powder (p-value = 0.201). As a liquid, the reconstituted powder was also highly bioactive, indicating that the excipient formulation can successfully protect the adenovirus during spray drying at sufficient viral potency for observable infection. There was no statistically significant difference between the average relative luciferase expression in reconstituted (p-value = 0.416), indicating that resulting bioactivity of these two treatments is comparable. Previous reports have shown that for reconstituted liquid and dry powder intratracheal delivery of spray dried siRNA and mRNA, luciferase luminescence in the lungs is often higher with reconstituted liquid delivery due to the challenges of insufflation in mice [7, 24]. Here we see that bioactivity was equivalently high after powder insufflation of our spray dried powder, further suggesting that there is strong powder dissolution within the distal lung to permit localized adenovirus infection and high luciferase expression. However, achieving dosage consistency between animals is still quite challenging with the pipette tip-loading dosator, leading to high variability in luciferase signals measured after dry powder delivery.
Average luciferase activity measured in mouse lung tissue three days following treatment. All treatments were delivered intratracheally including: placebo spray dried powder, reconstituted spray dried Ad5Hu5-Luc powder at 106 PFU and spray dried Ad5E1 CMV Luc powder at 106 PFU. Untreated mice are referred to as uninfected and serve as the negative control. Error bars represent the standard error between three animals (n = 3). Statistical difference (p-value < 0.05) between viral treatment groups and non-viral treatments is indicated as (*).
Conclusions
Selecting an intratracheal dosator for delivery of powder biopharmaceutics requires careful consideration of the materials of construction and powder loading method, especially for compressible vaccine powders with a high capacity for moisture absorption. Although the small scale of powder delivery to mice can be limiting, we have shown that syringe air volume and loaded mass have a significant influence on device performance and should therefore be optimized when developing a custom-made dosator. Overall, ambient moisture has a negative impact on device performance and the resulting powder dosage delivered suggesting that hydrophobic tip materials are preferred. Similarly, static charge can decrease the delivered amount of powder such that metals like stainless steel may offer advantages, especially for powders that are less moisture sensitive. In both cases, the rigidity of the tip physically impacts the ability to accurately align the dosator at the trachea and should be considered during device design. It is also suggested that after loading a dosator with moisture sensitive powder, the loaded assembly should be immediately stored on dry ice prior to in vivo administration. For highly compressible dry powders, dosators that use a pipette tip-loading method, rather than a chamber-loading or tamp-loading strategy, tend to minimize powder agglomeration or solidification to achieve sufficient emitted dose for distal lung delivery. Using the pipette tip-loading method, we have demonstrated for the first time that a thermally stable, spray dried adenovirus-containing dry powder can be delivered intratracheally in mice to yield a very strong bioactive response in the targeted lung tissue.
Based on this work, we highlight the unique challenges faced during murine pulmonary delivery and offer guidance on successful strategies that can improve intratracheal dosator performance when delivering compressible dry powder pharmaceutics. Due to the relationship between a viral vector and the effective formulation for its thermal stabilization as a powder, which affects important particle properties such as size, moisture sensitivity and flowability, future studies are warranted to expand our understanding on the design of a robust dosator.
References
Chaurasiya B, Zhao YY. Dry powder for pulmonary delivery: a comprehensive review. Pharmaceutics. 2021;13(1):1–28.
Kaur J, Muttil P, Verma RK, Kumar K, Yadav AB, Sharma R, et al. A hand-held apparatus for “nose-only” exposure of mice to inhalable microparticles as a dry powder inhalation targeting lung and airway macrophages. Eur J Pharm Sci. 2008;34(1):56–65.
Gomez M, Ahmed M, Das S, McCollum J, Mellett L, Swanson R, et al. Development and testing of a spray-dried tuberculosis vaccine candidate in a mouse model. Front Pharmacol. 2022;12(January):1–23.
Hickey AJ, Durham PG, Dharmadhikari A, Nardell EA. Inhaled drug treatment for tuberculosis: Past progress and future prospects. J Control Release [Internet]. 2016;240:127–34. Available from: https://doi.org/10.1016/j.jconrel.2015.11.018.
Rau JL. The inhalation of drugs: advantages and problems. Respir Care. 2005;50(3):367–82.
Manser MM, Feng X, Xing Z, Cranston ED, Thompson MR. Cryoprotective agents influence viral dosage and thermal stability of inhalable dry powder vaccines. Int J Pharm [Internet]. 2022;617 (December 2021):121602. Available from: https://doi.org/10.1016/j.ijpharm.2022.121602.
Qiu Y, Liao Q, Chow MYT, Lam JKW. Intratracheal administration of dry powder formulation in mice. J Vis Exp. 2020;2020(161):1–12.
Acosta A, Norazmi MN, Hernandez-Pando R, Alvarez N, Borrero R. Infante JF, et al. The importance of animal models in tuberculosis vaccine development. Malaysian J Med Sci. 2011;18(5):5–12.
Golding H, Khurana S, Zaitseva M. What is the predictive value of animal models for vaccine efficacy in humans? The importance of bridging studies and species-independent correlates of protection. Cold Spring Harb Perspect Biol. 2018;10(4):1–8.
Kuehl PJ, Anderson TL, Candelaria G, Gershman B, Harlin K, Hesterman JY, et al. Regional particle size dependent deposition of inhaled aerosols in rats and mice. Inhal Toxicol. 2012;24(1):27–35.
Durham PG, Hanif SN, Contreras LG, Young EF, Braunstein MS, Hickey AJ. Disposable dosators for pulmonary insufflation of therapeutic agents to small animals. J Vis Exp. 2017;2017(121):4–9.
Grimaud J, Murthy VN. How to monitor breathing in laboratory rodents: A review of the current methods. J Neurophysiol. 2018;120(2):624–32.
Ihara D, Hattori N, Horimasu Y, Masuda T, Nakashima T, Senoo T, et al. Histological quantification of gene silencing by intratracheal administration of dry powdered small-interfering RNA/Chitosan complexes in the murine lung. Pharm Res. 2015;32(12):3877–85.
Stewart IE, Lukka PB, Liu J, Meibohm B, Gonzalez-Juarrero M, Braunstein MS, et al. Development and characterization of a dry powder formulation for anti-tuberculosis drug spectinamide 1599. Pharm Res. 2019;36(9).
Manser M, Morgan BA, Feng X, Rhem RG, Dolovich MB, Xing Z, et al. Dextran mass ratio controls particle drying dynamics in a thermally stable dry powder vaccine for pulmonary delivery. Pharm Res [Internet]. 2022; Available from: https://doi.org/10.1007/s11095-022-03341-8.
Kishimoto K, Morimoto M. Mammalian tracheal development and reconstruction: Insights from in vivo and in vitro studies. Dev. 2021;148(13).
Irvin CG, Bates JHT. Measuring the lung function in the mouse: The challenge of size. Respir Res. 2003;4:1–9.
Jeyananthan V, Afkhami S, D’Agostino MR, Zganiacz A, Feng X, Miller MS, et al. Differential biodistribution of adenoviral-vectored vaccine following intranasal and endotracheal deliveries leads to different immune outcomes. Front Immunol. 2022;13(June):1–10.
Nyberg-Hoffman C, Aguilar-Cordova E. Instability of adenoviral vectors during transport and its implication for clinical studies. Nat Med. 1999;5(8):955–7.
Murtomaa M, Savolainen M, Christiansen L, Rantanen J, Laine E, Yliruusi J. Static electrification of powders during spray drying. J Electrostat. 2004;62(1):63–72.
Chung TCM. Functionalization of polypropylene with high dielectric properties: applications in electric energy storage. Green Sustain Chem. 2012;02(02):29–37.
Mehanna MM, Mohyeldin SM, Elgindy NA. Rifampicin-carbohydrate spray-dried nanocomposite: a futuristic multiparticulate platform for pulmonary delivery. Int J Nanomedicine. 2019;14:9089–112.
Zhang XR, Yamaguchi H. An experimental study on heat transfer of CO2 solid-gas two phase flow with dry ice sublimation. Int J Therm Sci [Internet]. 2011;50(11):2228–34. Available from: https://doi.org/10.1016/j.ijthermalsci.2011.05.019.
Qiu Y, Man RCH, Liao Q, Kung KLK, Chow MYT, Lam JKW. Effective mRNA pulmonary delivery by dry powder formulation of PEGylated synthetic KL4 peptide. J Control Release [Internet]. 2019;314(September):102–15. Available from: https://doi.org/10.1016/j.jconrel.2019.10.026.
Acknowledgements
This study was funded by the Canadian Institutes of Health Research (CIHR) and the Natural Sciences and Engineering Research Council of Canada (NSERC). The authors declare no conflicts of interest in this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Manser, M., Jeyanathan, V., Jeyanathan, M. et al. Design Considerations for Intratracheal Delivery Devices to Achieve Proof-of-Concept Dry Powder Biopharmaceutical Delivery in Mice. Pharm Res 40, 1165–1176 (2023). https://doi.org/10.1007/s11095-023-03492-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03492-2