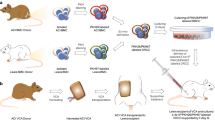
Abstract
Aim
Widespread clinical application of vascularized composite allotransplantation (VCA) has been limited by the need for lifelong systemic immunosuppression to prevent rejection. Our goal was to develop a site-specific immunosuppressive strategy that promotes VCA allograft survival and minimizes the risk of systemic side effects.
Methods
Tacrolimus loaded polycaprolactone (TAC-PCL) disks were prepared and tested for their efficacy in sustaining VCA allograft survival via site-specific immunosuppression. Brown Norway-to-Lewis rat hind limb transplantations were performed; animals received one TAC disk either in the transplanted (DTx) or in the contralateral non-transplanted (DnonTx) limbs. In another group, animals received DTx and lymphadenectomy on Tx side. Blood and allograft levels of TAC were measured using LC–MS/MS. Systemic toxicity was evaluated.
Results
Animals that received DTx achieved long-term allograft survival (> 200 days) without signs of metabolic and infectious complications. In these animals, TAC blood levels were low but stable between 2 to 5 ng/mL for nearly 100 days. High concentrations of TAC were achieved in the allografts and the draining lymph nodes (DLN). Animals that underwent lymphadenectomy rejected their allograft by 175 days. Animals that received DnonTx rejected their allografts by day 70.
Conclusion
Controlled delivery of TAC directly within the allograft (with a single TAC disk) effectively inhibits rejection and prolongs VCA allograft survival, while mitigating the complications of systemic immunosuppression. There was a survival benefit of delivering TAC within the allograft as compared to a remote site. We believe this approach of local drug delivery has significant implications for drug administration in transplantation.








Similar content being viewed by others
References
Ravindra KV, Ildstad ST. Immunosuppressive protocols and immunological challenges related to hand transplantation. Hand Clin. 2011;27(4):467–79, ix.
Sengoku T, et al. FK506 inhibition of histamine release and cytokine production by mast cells and basophils. Int J Immunopharmacol. 2000;22(3):189–201.
Fung JJ. Tacrolimus and transplantation: a decade in review. Transplantation. 2004;77(9 Suppl):S41–3.
Howsare M, Jones CM, Ramirez AM. Immunosuppression maintenance in vascularized composite allotransplantation: what is just right? Curr Opin Organ Transplant. 2017;22(5):463–9.
Varghese J, et al. Tacrolimus-related adverse effects in liver transplant recipients: its association with trough concentrations. Indian J Gastroenterol. 2014;33(3):219–25.
Bottiger Y, et al. Tacrolimus whole blood concentrations correlate closely to side-effects in renal transplant recipients. Br J Clin Pharmacol. 1999;48(3):445–8.
Ojo AO, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349(10):931–40.
Sui W, et al. Clinical study of the risk factors of insulin resistance and metabolic syndrome after kidney transplantation. Transpl Immunol. 2008;20(1–2):95–8.
Nadig SN. Transplant Arteriosclerosis. Transplantation. 2016;100(11):2249–50.
Lakkis FG, et al. Immunologic “ignorance” of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat Med. 2000;6(6):686–8.
Bai Y, et al. L-selectin-dependent lymphoid occupancy is required to induce alloantigen-specific tolerance. J Immunol. 2002;168(4):1579–89.
Golshayan D, et al. In vitro-expanded donor alloantigen-specific CD4+CD25+ regulatory T cells promote experimental transplantation tolerance. Blood. 2007;109(2):827–35.
Schnider JT, et al. Site-specific immunosuppression in vascularized composite allotransplantation: prospects and potential. Clin Dev Immunol. 2013;2013: 495212.
Solari MG, et al. Daily topical tacrolimus therapy prevents skin rejection in a rodent hind limb allograft model. Plast Reconstr Surg. 2009;123(2 Suppl):17S-25S.
Dean WK, Talbot SG. Vascularized Composite Allotransplantation at a Crossroad: Adopting Lessons From Technology Innovation to Novel Clinical Applications. Transplantation. 2017;101(3):452–6.
Gharb BB, et al. Effectiveness of topical immunosuppressants in prevention and treatment of rejection in face allotransplantation. Transplantation. 2013;95(10):1197–203.
Zamorano-Leon JJ, et al. New strategy of tacrolimus administration in animal model based on tacrolimus-loaded microspheres. Transpl Immunol. 2016;36:9–13.
Dane KY, et al. Nano-sized drug-loaded micelles deliver payload to lymph node immune cells and prolong allograft survival. J Control Release. 2011;156(2):154–60.
Olariu R, et al. Intra-graft injection of tacrolimus promotes survival of vascularized composite allotransplantation. J Surg Res. 2017;218:49–57.
Gerecke C, et al. Biocompatibility and characterization of polyglycerol-based thermoresponsive nanogels designed as novel drug-delivery systems and their intracellular localization in keratinocytes. Nanotoxicology. 2017;11(2):267–77.
Gajanayake T, et al. A single localized dose of enzyme-responsive hydrogel improves long-term survival of a vascularized composite allograft. Sci Transl Med. 2014;6(249):249ra110.
Unadkat JV, et al. Single Implantable FK506 Disk Prevents Rejection in Vascularized Composite Allotransplantation. Plast Reconstr Surg. 2017;139(2):403e–14e.
Sultana T, et al. Preparation and characterization of polycaprolactone-polyethylene glycol methyl ether and polycaprolactone-chitosan electrospun mats potential for vascular tissue engineering. J Biomater Appl. 2017;32(5):648–62.
Unadkat JV, et al. Composite tissue vasculopathy and degeneration following multiple episodes of acute rejection in reconstructive transplantation. Am J Transplant. 2010;10(2):251–61.
Cendales LC, et al. The Banff 2007 working classification of skin-containing composite tissue allograft pathology. Am J Transplant. 2008;8(7):1396–400.
Kambham N, et al. A novel, semiquantitative, clinically correlated calcineurin inhibitor toxicity score for renal allograft biopsies. Clin J Am Soc Nephrol. 2007;2(1):135–42.
Rodriguez-Rodriguez AE, et al. The higher diabetogenic risk of tacrolimus depends on pre-existing insulin resistance. A study in obese and lean Zucker rats. Am J Transplant. 2013;13(7):1665–75.
Kirresh T, et al. Development and validation of a liquid chromatography-mass spectrometric assay for simultaneous determination of tacrolimus and 13-O-desmethyl tacrolimus in rat kidney tissue. J Pharm Biomed Anal. 2017;136:32–7.
Eniola AO, Rodgers SD, Hammer DA. Characterization of biodegradable drug delivery vehicles with the adhesive properties of leukocytes. Biomaterials. 2002;23(10):2167–77.
Dailey LA, et al. Investigation of the proinflammatory potential of biodegradable nanoparticle drug delivery systems in the lung. Toxicol Appl Pharmacol. 2006;215(1):100–8.
Eniola AO, Hammer DA. Characterization of biodegradable drug delivery vehicles with the adhesive properties of leukocytes II: effect of degradation on targeting activity. Biomaterials. 2005;26(6):661–70.
Kanatani T, et al. Experimental limb transplantation, part III: induction of tolerance in the rigorous strain combination of Brown Norway donor to Lewis recipient. Transplant Proc. 2004;36(10):3276–82.
Schneeberger S, Khalifian S, Brandacher G. Immunosuppression and monitoring of rejection in hand transplantation. Tech Hand Up Extrem Surg. 2013;17(4):208–14.
Schweizer R, et al. Adipose-derived stromal cell therapy combined with a short course nonmyeloablative conditioning promotes long-term graft tolerance in vascularized composite allotransplantation. Am J Transplant. 2020;20(5):1272–84.
Thomson AW, Bonham CA, Zeevi A. Mode of action of tacrolimus (FK506): molecular and cellular mechanisms. Ther Drug Monit. 1995;17(6):584–91.
Laskow DA, et al. An open-label, concentration-ranging trial of FK506 in primary kidney transplantation: a report of the United States Multicenter FK506 Kidney Transplant Group. Transplantation. 1996;62(7):900–5.
Wingard JR, et al. Relationship of tacrolimus (FK506) whole blood concentrations and efficacy and safety after HLA-identical sibling bone marrow transplantation. Biol Blood Marrow Transplant. 1998;4(3):157–63.
Brenner MJ, et al. The spectrum of complications of immunosuppression: is the time right for hand transplantation? J Bone Joint Surg Am. 2002;84-A(10):1861–70.
Nielsen FT, et al. Kidney function and morphology after short-term combination therapy with cyclosporine A, tacrolimus and sirolimus in the rat. Nephrol Dial Transplant. 2003;18(3):491–6.
Nassar CA, et al. Biochemical evaluation of glycemic levels of long-term tacrolimus therapy in rats. Braz Oral Res. 2007;21(4):293–7.
Malinowski M, et al. Effect of tacrolimus dosing on glucose metabolism in an experimental rat model. Ann Transplant. 2010;15(3):60–5.
Acknowledgements
We thank Drs. Ernest Manders (University of Pittsburgh School of Medicine), Fadi Lakkis (Thomas Starzle Institute), and Taylor Skelly (University of Pittsburgh School of Pharmacy) for providing input and advice to our work; Spiros Giannoutsos, Shirley Tylor, and Joshua Sailor at the Clinical Laboratory Building, Special Chemistry Laboratory for aiding in samples analysis, and Sean Mcclaine for providing technical assistance with the figures. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This study was supported by intramural support from the Department of Plastic and Reconstructive Surgery and Department of Pharmaceutical Sciences, University of Pittsburgh. None of the authors have any conflicts of interest that may affect the content of the study.
Author information
Authors and Affiliations
Contributions
F.G.F., J.V.U., W.Z., J.A.P., V.S.G., K.M.W., M.G.S., R.V., and A.M.S. designed research; F.G.F., J.V.U., W.Z., Y.W., C.K., D.G., Z.Z., V.E.E., H.S., S.M and S.O. performed research; F.G.F.,R.V contributed new reagents/analytic tools; F.G.F., J.V.U., W.Z., M. E., J.A.P., V.S.G., K.M.W., M.G.S., R.V.R., and A.M.S. analyzed data; and F.G.F wrote the first draft and F.G.F. M.G.S., R.V.R., and A.M.S. provided feedback and revised the manuscript.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Feturi, F.G., Unadkat, J.V., Zhang, W. et al. Tacrolimus-Eluting Disk within the Allograft Enables Vascularized Composite Allograft Survival with Site-Specific Immunosuppression without Systemic Toxicity. Pharm Res 39, 2179–2190 (2022). https://doi.org/10.1007/s11095-022-03345-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03345-4