Abstract
Purpose
Increasing the efficiency of unsuccessful immunotherapy methods is one of the most important research fields. Therefore, the use of combination therapy is considered as one of the ways to increase the effectiveness of the dendritic cell (DC) vaccine. In this study, the inhibition of immune checkpoint receptors such as LAG3 and PD-1 on T cells was investigated to increase the efficiency of T cells in response to the DC vaccine.
Methods
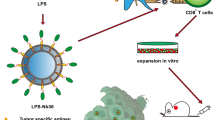
We used trimethyl chitosan-dextran sulfate-lactate (TMC-DS-L) nanoparticles (NPs) loaded with siRNA molecules to quench the PD-1 and LAG3 checkpoints’ expression.
Results
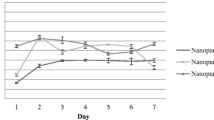
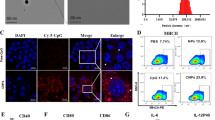
Appropriate physicochemical characteristics of the generated NPs led to efficient inhibition of LAG3 and PD-1 on T cells, which was associated with increased survival and activity of T cells, ex vivo. Also, treating mice with established breast tumors (4T1) using NPs loaded with siRNA molecules in combination with DC vaccine pulsed with tumor lysate significantly inhibited tumor growth and increased survival in mice. These ameliorative effects were associated with increased anti-tumor T cell responses and downregulation of immunosuppressive cells in the tumor microenvironment and spleen.
Conclusion
These findings strongly suggest that TMC-DS-L NPs loaded with siRNA could act as a novel tool in inhibiting the expression of immune checkpoints in the tumor microenvironment. Also, combination therapy based on inhibition of PD-1 and LAG3 in combination with DC vaccine is an effective method in treating cancer that needs to be further studied.






Similar content being viewed by others
Data Availability
All relevant information is presented within the article. Additional data that support the findings of this study are available from the corresponding author upon reasonable request.
Change history
26 December 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s11095-023-03647-1
References
Boer MC, Joosten SA, Ottenhoff THM. Regulatory T-cells at the interface between human host and pathogens in infectious diseases and vaccination. Front Immunol. 2015;6:217.
Gilboa E. Dendritic cell based vaccines. J Clin Invest. 2007;117:1195–203.
Karpisheh V, Mousavi SM, Sheykholeslami PN, Fathi M, Saray MM, Aghebati-Maleki L, et al. The role of regulatory T cells in the pathogenesis and treatment of prostate cancer. Life Sciences. 2021:119132.
Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4(3):321–7.
Lesterhuis WJ, Haanen JBAG, Punt CJA. Cancer immunotherapy–revisited. Nat Rev Drug Discovery. 2011;10(8):591–600.
Steven A, Rosenberg JCY, Nicholas P. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15.
Kazemi T, Younesi V, Jadidi-Niaragh F, Yousefi M. Immunotherapeutic approaches for cancer therapy: An updated review. Artif Cells Nanomed Biotechnol. 2016;44(3):769–79.
Fathi M, Bahmanpour S, Barshidi A, Rasouli H, Kiani FK, Khesht AMS, et al. Simultaneous blockade of TIGIT and HIF-1α induces synergistic anti-tumor effect and decreases the growth and development of cancer cells. Int Immunopharmacol. 2021;101: 108288.
Fathi M, Pustokhina I, Kuznetsov SV, Khayrullin M, Hojjat-Farsangi M, Karpisheh V, et al. T-cell immunoglobulin and ITIM domain, as a potential immune checkpoint target for immunotherapy of colorectal cancer. IUBMB Life. 2021;73(5):726–38.
Pawłowska A, Suszczyk D, Okła K, Barczyński B, Kotarski J, Wertel I. Immunotherapies based on PD-1/PD-L1 pathway inhibitors in ovarian cancer treatment. Clin Exp Immunol. 2019;195(3):334–44.
Karpisheh V, Ahmadi M, Abbaszadeh-Goudarzi K, Mohammadpour Saray M, Barshidi A, Mohammadi H, et al. The role of Th17 cells in the pathogenesis and treatment of breast cancer. Cancer Cell Int. 2022;22(1):1–13.
Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther. 2015;37(4):764–82.
Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974.
Bastaki S, Aravindhan S, Ahmadpour Saheb N, Afsari Kashani M, Evgenievich Dorofeev A, Karoon Kiani F, et al. Codelivery of STAT3 and PD-L1 siRNA by hyaluronate-TAT trimethyl/thiolated chitosan nanoparticles suppresses cancer progression in tumor-bearing mice. Life Sci. 2021;266: 118847.
Esmaily M, Masjedi A, Hallaj S, Nabi Afjadi M, Malakotikhah F, Ghani S, et al. Blockade of CTLA-4 increases anti-tumor response inducing potential of dendritic cell vaccine. Journal of controlled release : official journal of the Controlled Release Society. 2020;326:63–74.
Ghasemi-Chaleshtari M, Kiaie SH, Irandoust M, Karami H, Nabi Afjadi M, Ghani S, et al. Concomitant blockade of A2AR and CTLA-4 by siRNA-loaded polyethylene glycol-chitosan-alginate nanoparticles synergistically enhances antitumor T-cell responses. J Cell Physiol. 2020;235(12):10068–80.
Masjedi A, Ahmadi A, Ghani S, Malakotikhah F, Nabi Afjadi M, Irandoust M, et al. Silencing adenosine A2a receptor enhances dendritic cell-based cancer immunotherapy. Nanomed Nanotechnol Biol Med. 2020;29: 102240.
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34.
Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203(4):883–95.
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7–H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800.
Fanoni D, Tavecchio S, Recalcati S, Balice Y, Venegoni L, Fiorani R, et al. New monoclonal antibodies against B-cell antigens: possible new strategies for diagnosis of primary cutaneous B-cell lymphomas. Immunol Lett. 2011;134(2):157–60.
Puhr HC, Ilhan-Mutlu A. New emerging targets in cancer immunotherapy: the role of LAG3. ESMO open. 2019;4(2): e000482.
Yazdani Y, Mohammadnia-Afrouzi M, Yousefi M, Anvari E, Ghalamfarsa G, Hasannia H, et al. Myeloid-derived suppressor cells in B cell malignancies. Tumor Biology. 2015;36(10):7339–53.
Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3—potential mechanisms of action. Nat Rev Immunol. 2015;15(1):45–56.
Nada MH, Wang H, Hussein AJ, Tanaka Y, Morita CT. PD-1 checkpoint blockade enhances adoptive immunotherapy by human Vγ2Vδ2 T cells against human prostate cancer. Oncoimmunology. 2021;10(1):1989789.
Zahm CD, Moseman JE, Delmastro LE, D GM. PD-1 and LAG-3 blockade improve anti-tumor vaccine efficacy. Oncoimmunology. 2021;10(1):1912892.
Jiang H, Ni H, Zhang P, Guo X, Wu M, Shen H, et al. PD-L1/LAG-3 bispecific antibody enhances tumor-specific immunity. Oncoimmunology. 2021;10(1):1943180.
Huang R-Y, Francois A, McGray AJR, Miliotto A, Odunsi K. Compensatory upregulation of PD-1, LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in metastatic ovarian cancer. Oncoimmunology. 2017;6(1): e1249561.
Okazaki T, Okazaki I-m, Wang J, Sugiura D, Nakaki F, Yoshida T, et al. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. Journal of Experimental Medicine. 2011;208(2):395–407.
Hajizadeh F, Ardebili SM, Moornani MB, Masjedi A, Atyabi F, Kiani M, et al. Silencing of HIF-1α/CD73 axis by siRNA-loaded TAT-chitosan-spion nanoparticles robustly blocks cancer cell progression. Eur J Pharmacol. 2020;882: 173235.
Joshi N, Hajizadeh F, Dezfouli EA, Zekiy AO, Afjadi MN, Mousavi SM, et al. Silencing STAT3 enhances sensitivity of cancer cells to doxorubicin and inhibits tumor progression. Life Sci. 2021;275: 119369.
Khesht AMS, Karpisheh V, Gilan PS, Melnikova LA, Zekiy AO, Mohammadi M, et al. Blockade of CD73 using siRNA loaded chitosan lactate nanoparticles functionalized with TAT-hyaluronate enhances doxorubicin mediated cytotoxicity in cancer cells both in vitro and in vivo. Int J Biol Macromol. 2021;186:849–63.
Dang Y, Guan J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Materials in Medicine. 2020;1:10–9.
Bahrami B, Hojjat-Farsangi M, Mohammadi H, Anvari E, Ghalamfarsa G, Yousefi M, et al. Nanoparticles and targeted drug delivery in cancer therapy. Immunol Lett. 2017;190:64–83.
Hashemi V, Farhadi S, Ghasemi Chaleshtari M, Seashore-Ludlow B, Masjedi A, Hojjat-Farsangi M, et al. Nanomedicine for improvement of dendritic cell-based cancer immunotherapy. Int Immunopharmacol. 2020;83: 106446.
Mourya VK, Inamdar NN. Trimethyl chitosan and its applications in drug delivery. J Mater Sci - Mater Med. 2009;20(5):1057–79.
Tiyaboonchai W, Limpeanchob N. Formulation and characterization of amphotericin B–chitosan–dextran sulfate nanoparticles. Int J Pharm. 2007;329(1–2):142–9.
Karpisheh V, Joshi N, Zekiy AO, Beyzai B, Hojjat-Farsangi M, Namdar A, et al. EP4 Receptor as a Novel Promising Therapeutic Target in Colon Cancer: Running title: EP4 receptor in colon cancer. Pathology-Research and Practice. 2020:153247.
Weecharangsan W, Opanasopit P, Ngawhirunpat T, Rojanarata T, Apirakaramwong A. Chitosan lactate as a nonviral gene delivery vector in COS-1 cells. AAPS PharmSciTech. 2006;7(3):E74–9.
Sarmento B, Martins S, Ribeiro A, Veiga F, Neufeld R, Ferreira D. Development and comparison of different nanoparticulate polyelectrolyte complexes as insulin carriers. Int J Pept Res Ther. 2006;12(2):131–8.
Eivazy P, Atyabi F, Jadidi-Niaragh F, Aghebati Maleki L, Miahipour A, Abdolalizadeh J, et al. The impact of the codelivery of drug-siRNA by trimethyl chitosan nanoparticles on the efficacy of chemotherapy for metastatic breast cancer cell line (MDA-MB-231). Artificial cells, nanomedicine, and biotechnology. 2017;45(5):889–96.
Hashemi V, Ahmadi A, Malakotikhah F, Chaleshtari MG, Baghi Moornani M, Masjedi A, et al. Silencing of p68 and STAT3 synergistically diminishes cancer progression. Life Sci. 2020;249: 117499.
Hallaj S, Heydarzadeh Asl S, Alian F, Farshid S, Eshaghi FS, Namdar A, et al. Inhibition of CD73 using folate targeted nanoparticles carrying anti-CD73 siRNA potentiates anticancer efficacy of Dinaciclib. Life Sci. 2020;259: 118150.
Alzamely KO, Hajizadeh F, Heydari M, Ghaderi Sede MJ, Asl SH, Peydaveisi M, et al. Combined inhibition of CD73 and ZEB1 by Arg-Gly-Asp (RGD)-targeted nanoparticles inhibits tumor growth. Colloids Surf, B. 2021;197: 111421.
Jadidi-Niaragh F, Atyabi F, Rastegari A, Mollarazi E, Kiani M, Razavi A, et al. Downregulation of CD73 in 4T1 breast cancer cells through siRNA-loaded chitosan-lactate nanoparticles. Tumor Biology. 2016;37(6):8403–12.
Allahyari SE, Hajizadeh F, Zekiy AO, Mansouri N, Gilan PS, Mousavi SM, et al. Simultaneous inhibition of CD73 and IL-6 molecules by siRNA-loaded nanoparticles prevents the growth and spread of cancer. Nanomed Nanotechnol Biol Med. 2021;34: 102384.
Fathi M, Bahmanpour S, Barshidi A, Rasouli H, Karoon Kiani F, Mahmoud Salehi Khesht A, et al. Simultaneous blockade of TIGIT and HIF-1α induces synergistic anti-tumor effect and decreases the growth and development of cancer cells. Int Immunopharmacol. 2021;101(Pt A):108288.
Hosseini F, Hassannia H, Mahdian-Shakib A, Jadidi-Niaragh F, Enderami SE, Fattahi M, et al. Targeting of crosstalk between tumor and tumor microenvironment by β-D mannuronic acid (M2000) in murine breast cancer model. Cancer Med. 2017;6(3):640–50.
Jadidi-Niaragh F, Yousefi M, Memarian A, Hojjat-Farsangi M, Khoshnoodi J, Razavi SM, et al. Increased frequency of CD8+ and CD4+ regulatory T cells in chronic lymphocytic leukemia: association with disease progression. Cancer Invest. 2013;31(2):121–31.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75.
Hassannia H, Ghasemi Chaleshtari M, Atyabi F, Nosouhian M, Masjedi A, Hojjat-Farsangi M, et al. Blockage of immune checkpoint molecules increases T-cell priming potential of dendritic cell vaccine. Immunology. 2020;159(1):75–87.
Ghalamfarsa G, Jadidi-Niaragh F, Hojjat-Farsangi M, Asgarian-Omran H, Yousefi M, Tahmasebi F, et al. Differential regulation of B-cell proliferation by IL21 in different subsets of chronic lymphocytic leukemia. Cytokine. 2013;62(3):439–45.
Jadidi-Niaragh F, Jeddi-Tehrani M, Ansaripour B, Razavi SM, Sharifian RA, Shokri F. Reduced frequency of NKT-like cells in patients with progressive chronic lymphocytic leukemia. Medical oncology (Northwood, London, England). 2012;29(5):3561–9.
Esmaily M, Masjedi A, Hallaj S, Afjadi MN, Malakotikhah F, Ghani S, et al. Blockade of CTLA-4 increases anti-tumor response inducing potential of dendritic cell vaccine. J Control Release. 2020;326:63–74.
Ghasemi-Chaleshtari M, Kiaie SH, Irandoust M, Karami H, Nabi Afjadi M, Ghani S, et al. Concomitant blockade of A2AR and CTLA-4 by siRNA-loaded polyethylene glycol-chitosan-alginate nanoparticles synergistically enhances antitumor T-cell responses. J Cell Physiol. 2020;235(12):10068–80.
Hosseini F, Mahdian-Shakib A, Jadidi-Niaragh F, Enderami SE, Mohammadi H, Hemmatzadeh M, et al. Anti-inflammatory and anti-tumor effects of α-l-guluronic acid (G2013) on cancer-related inflammation in a murine breast cancer model. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2018;98:793–800.
Hajizadeh F, Okoye I, Esmaily M, Ghasemi Chaleshtari M, Masjedi A, Azizi G, et al. Hypoxia inducible factors in the tumor microenvironment as therapeutic targets of cancer stem cells. Life Sci. 2019;237: 116952.
Haji-Fatahaliha M, Hosseini M, Akbarian A, Sadreddini S, Jadidi-Niaragh F, Yousefi M. CAR-modified T-cell therapy for cancer: an updated review. Artificial cells, nanomedicine, and biotechnology. 2016;44(6):1339–49.
Burugu S, Gao D, Leung S, Chia SK, Nielsen TO. LAG-3+ tumor infiltrating lymphocytes in breast cancer: clinical correlates and association with PD-1/PD-L1+ tumors. Ann Oncol. 2017;28(12):2977–84.
Woo S-R, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Can Res. 2012;72(4):917–27.
Bastaki S, Irandoust M, Ahmadi A, Hojjat-Farsangi M, Ambrose P, Hallaj S, et al. PD-L1/PD-1 axis as a potent therapeutic target in breast cancer. Life Sci. 2020;247: 117437.
Kiani FK, Izadi S, Dezfouli EA, Ebrahimi F, Mohammadi M, Chalajour H, et al. Simultaneous silencing of the A2aR and PD-1 immune checkpoints by siRNA-loaded nanoparticles enhances the immunotherapeutic potential of dendritic cell vaccine in tumor experimental models. Life Sci. 2022;288: 120166.
Leone RD, Sun I-M, Oh M-H, Sun I-H, Wen J, Englert J, et al. Inhibition of the adenosine A2a receptor modulates expression of T cell coinhibitory receptors and improves effector function for enhanced checkpoint blockade and ACT in murine cancer models. Cancer Immunol Immunother. 2018;67(8):1271–84.
Wu Z, Yang L, Shi L, Song H, Shi P, Yang T, et al. Prognostic impact of adenosine receptor 2 (A2aR) and programmed cell death ligand 1 (PD-L1) expression in colorectal cancer. BioMed research international. 2019;2019.
Andrews LP, Marciscano AE, Drake CG, Vignali DAA. LAG 3 (CD 223) as a cancer immunotherapy target. Immunol Rev. 2017;276(1):80–96.
Goldberg MV, Drake CG. LAG-3 in cancer immunotherapy. Cancer Immunology and Immunotherapy. 2010:269–78.
Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10(1):29–37.
Perez-Santos M, Anaya-Ruiz M, Cebada J, Bandala C, Landeta G, Martínez-Morales P, et al. LAG-3 antagonists by cancer treatment: a patent review. Expert Opin Ther Pat. 2019;29(8):643–51.
Webster RM. The immune checkpoint inhibitors: where are we now? Nat Rev Drug Discovery. 2014;13(12):883.
Hassannia H, Ghasemi Chaleshtari M, Atyabi F, Nosouhian M, Masjedi A, Hojjat-Farsangi M, et al. Blockage of immune checkpoint molecules increases T-cell priming potential of dendritic cell vaccine. Immunology. 2020;159(1):75–87.
Brignone C, Gutierrez M, Mefti F, Brain E, Jarcau R, Cvitkovic F, et al. First-line chemoimmunotherapy in metastatic breast carcinoma: combination of paclitaxel and IMP321 (LAG-3Ig) enhances immune responses and antitumor activity. J Transl Med. 2010;8(1):1–11.
Han Q, Wang Y, Pang M, Zhang J. STAT3-blocked whole-cell hepatoma vaccine induces cellular and humoral immune response against HCC. J Exp Clin Cancer Res. 2017;36(1):1–11.
Zhou G, Sprengers D, Boor PPC, Doukas M, Schutz H, Mancham S, et al. Antibodies against immune checkpoint molecules restore functions of tumor-infiltrating T cells in hepatocellular carcinomas. Gastroenterology. 2017;153(4):1107–19.
Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378(21):1976–86.
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71.
Yoshida K, Okamoto M, Sasaki J, Kuroda C, Ishida H, Ueda K, et al. Anti-PD-1 antibody decreases tumour-infiltrating regulatory T cells. BMC Cancer. 2020;20(1):1–10.
Du H, Yi Z, Wang L, Li Z, Niu B, Ren G. The co-expression characteristics of LAG3 and PD-1 on the T cells of patients with breast cancer reveal a new therapeutic strategy. Int Immunopharmacol. 2020;78: 106113.
Luo F, Cao J, Lu F, Zeng K, Ma W, Huang Y, et al. Lymphocyte activating gene 3 protein expression in nasopharyngeal carcinoma is correlated with programmed cell death-1 and programmed cell death ligand-1, tumor-infiltrating lymphocytes. Cancer Cell Int. 2021;21(1):1–16.
Kiani FK, Izadi S, Dezfouli EA, Ebrahimi F, Mohammadi M, Chalajour H, et al. Simultaneous silencing of A2aR and PD-1 as immune checkpoints by siRNA-loaded nanoparticles enhances the immunotherapeutic potential of dendritic cell vaccine in tumor experimental models. Life Sciences. 2021:120166.
Budi HS, Izadi S, Timoshin A, Asl SH, Beyzai B, Ghaderpour A, et al. Blockade of HIF-1α and STAT3 by hyaluronate-conjugated TAT-chitosan-SPION nanoparticles loaded with siRNA molecules prevents tumor growth. Nanomed Nanotechnol Biol Med. 2021;34: 102373.
Nikkhoo A, Rostami N, Farhadi S, Esmaily M, Moghadaszadeh Ardebili S, Atyabi F, et al. Codelivery of STAT3 siRNA and BV6 by carboxymethyl dextran trimethyl chitosan nanoparticles suppresses cancer cell progression. Int J Pharm. 2020;581: 119236.
Rostami N, Nikkhoo A, Khazaei-Poul Y, Farhadi S, Sadat Haeri M, Moghadaszadeh Ardebili S, et al. Coinhibition of S1PR1 and GP130 by siRNA-loaded alginate-conjugated trimethyl chitosan nanoparticles robustly blocks development of cancer cells. J Cell Physiol. 2020;235(12):9702–17.
Masjedi A, Ahmadi A, Atyabi F, Farhadi S, Irandoust M, Khazaei-Poul Y, et al. Silencing of IL-6 and STAT3 by siRNA loaded hyaluronate-N, N, N-trimethyl chitosan nanoparticles potently reduces cancer cell progression. Int J Biol Macromol. 2020;149:487–500.
Onyebuchi C, Kavaz D. Chitosan and N, N, N-Trimethyl chitosan nanoparticle encapsulation of Ocimum gratissimum essential oil: Optimised synthesis, in vitro release and bioactivity. Int J Nanomed. 2019;14:7707.
Slütter B, Bal S, Keijzer C, Mallants R, Hagenaars N, Que I, et al. Nasal vaccination with N-trimethyl chitosan and PLGA based nanoparticles: nanoparticle characteristics determine quality and strength of the antibody response in mice against the encapsulated antigen. Vaccine. 2010;28(38):6282–91.
Ghosh S, Sharma G, Travers J, Kumar S, Choi J, Jun HT, et al. TSR-033, a novel therapeutic antibody targeting LAG-3, enhances T-cell function and the activity of PD-1 blockade in vitro and in vivo. Mol Cancer Ther. 2019;18(3):632–41.
Zhang Q, Chikina M, Szymczak-Workman AL, Horne W, Kolls JK, Vignali KM, et al. LAG-3 limits regulatory T cell proliferation and function in autoimmune diabetes. Science immunology. 2017;2(9).
Zhou G, Noordam L, Sprengers D, Doukas M, Boor PPC, van Beek AA, et al. Blockade of LAG3 enhances responses of tumor-infiltrating T cells in mismatch repair-proficient liver metastases of colorectal cancer. Oncoimmunology. 2018;7(7): e1448332.
Breton G, Yassine-Diab B, Cohn L, Boulassel M-R, Routy J-P, Sékaly R-P, et al. SiRNA knockdown of PD-L1 and PD-L2 in monocyte-derived dendritic cells only modestly improves proliferative responses to gag by CD8+ T cells from HIV-1-infected individuals. J Clin Immunol. 2009;29(5):637–45.
Wei J, Luo C, Wang Y, Guo Y, Dai H, Tong C, et al. PD-1 silencing impairs the anti-tumor function of chimeric antigen receptor modified T cells by inhibiting proliferation activity. J Immunother Cancer. 2019;7(1):1–15.
Foy SP, Sennino B, dela Cruz T, Cote JJ, Gordon EJ, Kemp F, et al. Poxvirus-based active immunotherapy with PD-1 and LAG-3 dual immune checkpoint inhibition overcomes compensatory immune regulation, yielding complete tumor regression in mice. PloS one. 2016;11(2):e0150084.
Goding SR, Wilson KA, Xie Y, Harris KM, Baxi A, Akpinarli A, et al. Restoring immune function of tumor-specific CD4+ T cells during recurrence of melanoma. J Immunol. 2013;190(9):4899–909.
Huang R-Y, Eppolito C, Lele S, Shrikant P, Matsuzaki J, Odunsi K. LAG3 and PD1 co-inhibitory molecules collaborate to limit CD8+ T cell signaling and dampen antitumor immunity in a murine ovarian cancer model. Oncotarget. 2015;6(29):27359.
Lichtenegger FS, Rothe M, Schnorfeil FM, Deiser K, Krupka C, Augsberger C, et al. Targeting LAG-3 and PD-1 to enhance T cell activation by antigen-presenting cells. Front Immunol. 2018;9:385.
Wölfl M, Greenberg PD. Antigen-specific activation and cytokine-facilitated expansion of naive, human CD8+ T cells. Nat Protoc. 2014;9(4):950–66.
Jadidi-Niaragh F, Mirshafiey A. Regulatory T-cell as orchestra leader in immunosuppression process of multiple sclerosis. Immunopharmacol Immunotoxicol. 2011;33(3):545–67.
Kheshti AMS, Hajizadeh F, Barshidi A, Rashidi B, Ebrahimi F, Bahmanpour S, et al. Combination Cancer Immunotherapy with Dendritic Cell Vaccine and Nanoparticles Loaded with Interleukin-15 and Anti-beta-catenin siRNA Significantly Inhibits Cancer Growth and Induces Anti-Tumor Immune Response. Pharm Res. 2022;39(2):353–67.
Antonios JP, Soto H, Everson RG, Orpilla J, Moughon D, Shin N, et al. PD-1 blockade enhances the vaccination-induced immune response in glioma. JCI insight. 2016;1(10).
Ge Y, Xi H, Ju S, Zhang X. Blockade of PD-1/PD-L1 immune checkpoint during DC vaccination induces potent protective immunity against breast cancer in hu-SCID mice. Cancer Lett. 2013;336(2):253–9.
ACKNOWLEDGMENTS AND DISCLOSURES
We are very grateful for the financial support of Urmia University of Medical Sciences (grant number:10954) and Tabriz University of Medical Sciences (grant numbers: 66472 and 66443) for this study. We also thank the National Institute for Medical Research Development, NIMAD, for supporting this study (grant number: 4000524). We would also like thank to Koosha Koorehpaz and Mehran Mozaffari for their excellent contribution in this study. There is no conflict of interest.
Author information
Authors and Affiliations
Contributions
Conceptualization: Asal Barshidi, Vahid Karpisheh, Mohammad Hojjat-Farsangi, Naime Majidi Zolbanin. Data curation: Asal Barshidi, Hadi Hassannia, Sanam Nami, Fariba Karoon Kiani, Negin Afsharimanesh, Jamshid Gholizadeh Navashenaq. Formal analysis: Asal Barshidi, Vahid Karpisheh, Fariba Karoon Kiani, Negin Afsharimanesh, Jamshid Gholizadeh Navashenaq, Ata Mahmoodpoor. Funding acquisition: Ata Mahmoodpoor, Reza Jafari, Farhad Jadidi-Niaragh, Fatemeh Karimian Noukabadi. Methodology: Asal Barshidi, Vahid Karpisheh, Hadi Hassannia, Jamshid Gholizadeh Navashenaq, Sanam Nami, Pooya Jalali1 Project administration: Farbod Ebrahimi, Seyed Hossein Kiaie, Reza Jafari, Farhad Jadidi-Niaragh.
Writing-review and editing: Reza Jafari, Farhad Jadidi-Niaragh.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s11095-023-03647-1
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barshidi, A., Karpisheh, V., Noukabadi, F.K. et al. RETRACTED ARTICLE: Dual Blockade of PD-1 and LAG3 Immune Checkpoints Increases Dendritic Cell Vaccine Mediated T Cell Responses in Breast Cancer Model. Pharm Res 39, 1851–1866 (2022). https://doi.org/10.1007/s11095-022-03297-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03297-9