Abstract
Purpose
Modulation of 5-HT3 receptor in the central nervous system (CNS) is a promising approach for treatment of neuropathic pain. The goal was to evaluate the role of P-glycoprotein (Pgp) in limiting exposure of different parts of the CNS to ondansetron (5-HT3 receptor antagonist) using wild-type and genetic knockout rat model.
Methods
Plasma pharmacokinetics and CNS (brain, spinal cord, and cerebrospinal fluid) disposition was studied after single 10 mg/kg intravenous dose.
Results
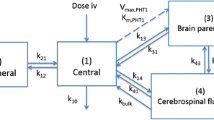
Pgp knockout resulted in significantly higher concentrations of ondansetron in all tested regions of the CNS at most of the time points. The mean ratio of the concentrations between KO and WT animals was 2.39–5.48, depending on the region of the CNS. Male and female animals demonstrated some difference in ondansetron plasma pharmacokinetics and CNS disposition. Mechanistic pharmacokinetic model that included two systemic disposition and three CNS compartments (with intercompartmental exchange) was developed. Pgp transport was incorporated as an efflux from the brain and spinal cord to the central compartment. The model provided good simultaneous description of all data sets, and all parameters were estimated with sufficient precision.
Conclusions
The study provides important quantitative information on the role of Pgp in limiting ondansetron exposure in various regions of the CNS using data from wild-type and Pgp knockout rats. CSF drug concentrations, as a surrogate to CNS exposure, are likely to underestimate the effect of Pgp on drug penetration to the brain and the spinal cord.




Similar content being viewed by others
References
van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155(4):654–62.
Attal N, Bouhassira D, Baron R. Diagnosis and assessment of neuropathic pain through questionnaires. Lancet Neurol. 2018;17(5):456–66.
Zilliox LA. Neuropathic Pain. Continuum (Minneap Minn). 2017;23(2, Selected Topics in Outpatient Neurology):512–32.
Finnerup NB, Attal N. Pharmacotherapy of neuropathic pain: time to rewrite the rulebook? Pain Manag. 2016;6(1):1–3.
Dogrul A, Ossipov MH, Porreca F. Differential mediation of descending pain facilitation and inhibition by spinal 5HT-3 and 5HT-7 receptors. Brain Res. 2009;1280:52–9.
Suzuki R, Rahman W, Hunt SP, Dickenson AH. Descending facilitatory control of mechanically evoked responses is enhanced in deep dorsal horn neurones following peripheral nerve injury. Brain Res. 2004;1019(1–2):68–76.
Oatway MA, Chen Y, Weaver LC. The 5-HT3 receptor facilitates at-level mechanical allodynia following spinal cord injury. Pain. 2004;110(1–2):259–68.
Bedford FK, Julius D, Ingraham HA. Neuronal expression of the 5HT3 serotonin receptor gene requires nuclear factor 1 complexes. J Neurosci. 1998;18(16):6186–94.
McCleane GJ, Suzuki R, Dickenson AH. Does a single intravenous injection of the 5HT3 receptor antagonist ondansetron have an analgesic effect in neuropathic pain? A double-blinded, placebo-controlled cross-over study. Anesth Analg. 2003;97(5):1474–8.
Zofran (ondansetron hydrochloride) Prescribing Information. Food and Drug Administration.
Kulsoom Farhat MI. Shabana Ali, Anwar Kamal pasha. Resistance to ondansetron: role of pharmacogenetics in post-operative nausea and vomiting. Egyptian Journal of Medical Human Genetics. 2013;14(4):331–6.
Ye JH, Ponnudurai R, Schaefer R. Ondansetron: a selective 5-HT(3) receptor antagonist and its applications in CNS-related disorders. CNS Drug Rev. 2001;7(2):199–213.
Perez EA. Review of the preclinical pharmacology and comparative efficacy of 5-hydroxytryptamine-3 receptor antagonists for chemotherapy-induced emesis. J Clin Oncol. 1995;13(4):1036–43.
Janelsins MC, Tejani MA, Kamen C, Peoples AR, Mustian KM, Morrow GR. Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opin Pharmacother. 2013;14(6):757–66.
Chatterjee S, Rudra A, Sengupta S. Current concepts in the management of postoperative nausea and vomiting. Anesthesiol Res Pract. 2011;2011:748031.
MacDougall MR, Sharma S. Physiology, chemoreceptor trigger zone. Treasure Island: StatPearls; 2019.
Koyama Y, Kondo M, Shimada S. Building a 5-HT3A receptor expression map in the mouse brain. Sci Rep. 2017;7:42884.
Schinkel AH, Wagenaar E, Mol CA, van Deemter L. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97(11):2517–24.
Lexicomp. Ondansetron - Pharmacology & Pharmacokinetics. Available from: https://online.lexi.com/.
Yang SH, Yang KH, Lee MG. Gender differences in ondansetron pharmacokinetics in rats. Biopharm Drug Dispos. 2008;29(7):406–13.
Pritchard JF, Bryson JC, Kernodle AE, Benedetti TL, Powell JR. Age and gender effects on ondansetron pharmacokinetics: evaluation of healthy aged volunteers. Clin Pharmacol Ther. 1992;51(1):51–5.
Jann MW, ZumBrunnen TL, Tenjarla SN, Ward ES Jr, Weidler DJ. Relative bioavailability of ondansetron 8-mg oral tablets versus two extemporaneous 16-mg suppositories: formulation and gender differences. Pharmacotherapy. 1998;18(2):288–94.
Chong YE, Chiang M, Deshpande K, Haroutounian S, Kagan L, Lee JB. Simultaneous quantification of ondansetron and tariquidar in rat and human plasma using a high performance liquid chromatography-ultraviolet method. Biomed Chromatogr 2019:e4653.
Depot M, Leroux S, Caille G. High-resolution liquid chromatographic method using ultraviolet detection for determination of ondansetron in human plasma. J Chromatogr B Biomed Sci Appl. 1997;693(2):399–406.
Yang SH, Lee MG. Dose-independent pharmacokinetics of ondansetron in rats: contribution of hepatic and intestinal first-pass effects to low bioavailability. Biopharm Drug Dispos. 2008;29(7):414–26.
NextAdvance. Brain - Bullet Blender Homogenization Protocol. Available from: https://www.nextadvance.us/scripts/public/bullet-blender/protocols/protocol_display/protocol.php?samplename=Brain&sample_id=10&protocol_id=15.
Wang XX, Li YB, Feng MR, Smith DE. Semi-mechanistic population pharmacokinetic modeling of L-Histidine disposition and brain uptake in Wildtype and Pht1 null mice. Pharm Res. 2018;35(1):19.
Gaohua L, Neuhoff S, Johnson TN, Rostami-Hodjegan A, Jamei M. Development of a permeability-limited model of the human brain and cerebrospinal fluid (CSF) to integrate known physiological and biological knowledge: estimating time varying CSF drug concentrations and their variability using in vitro data. Drug Metab Pharmacokinet. 2016;31(3):224–33.
Yamamoto Y, Valitalo PA, van den Berg DJ, Hartman R, van den Brink W, Wong YC, et al. A generic multi-compartmental CNS distribution model structure for 9 drugs allows prediction of human brain target site concentrations. Pharm Res. 2017;34(2):333–51.
Hammarlund-Udenaes M, Friden M, Syvanen S, Gupta A. On the rate and extent of drug delivery to the brain. Pharm Res. 2008;25(8):1737–50.
Kawakami J, Yamamoto K, Sawada Y, Iga T. Prediction of brain delivery of ofloxacin, a new quinolone, in the human from animal data. J Pharmacokinet Biopharm. 1994;22(3):207–27.
Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128(6):309–16.
Betts JG, Johnson E, Wise JA, Young KA. Anatomy and physiology (OpenStax); 2019.
Jablonski MR, Jacob DA, Campos C, Miller DS, Maragakis NJ, Pasinelli P, et al. Selective increase of two ABC drug efflux transporters at the blood-spinal cord barrier suggests induced pharmacoresistance in ALS. Neurobiol Dis. 2012;47(2):194–200.
Dulin JN, Moore ML, Grill RJ. The dual cyclooxygenase/5-lipoxygenase inhibitor licofelone attenuates p-glycoprotein-mediated drug resistance in the injured spinal cord. J Neurotrauma. 2013;30(3):211–26.
Harrold J. Available from: https://ubiquity.tools.
Svensson CI, Tran TK, Fitzsimmons B, Yaksh TL, Hua XY. Descending serotonergic facilitation of spinal ERK activation and pain behavior. FEBS Lett. 2006;580(28–29):6629–34.
Tuveson B, Leffler AS, Hansson P. Ondansetron, a 5HT3-antagonist, does not alter dynamic mechanical allodynia or spontaneous ongoing pain in peripheral neuropathy. Clin J Pain. 2011;27(4):323–9.
Hamidovic A, Hahn K, Kolesar J. Clinical significance of ABCB1 genotyping in oncology. J Oncol Pharm Pract. 2010;16(1):39–44.
Schinkel AH. P-glycoprotein, a gatekeeper in the blood-brain barrier. Adv Drug Deliv Rev. 1999;36(2–3):179–94.
Bundgaard C, Jensen CJ, Garmer M. Species comparison of in vivo P-glycoprotein-mediated brain efflux using mdr1a-deficient rats and mice. Drug Metab Dispos. 2012;40(3):461–6.
Huang L, Li X, Roberts J, Janosky B, Lin MH. Differential role of P-glycoprotein and breast cancer resistance protein in drug distribution into brain, CSF and peripheral nerve tissues in rats. Xenobiotica. 2015;45(6):547–55.
Zamek-Gliszczynski MJ, Bedwell DW, Bao JQ, Higgins JW. Characterization of SAGE Mdr1a (P-gp), Bcrp, and Mrp2 knockout rats using loperamide, paclitaxel, sulfasalazine, and carboxydichlorofluorescein pharmacokinetics. Drug Metab Dispos. 2012;40(9):1825–33.
Syvanen S, Hooker A, Rahman O, Wilking H, Blomquist G, Langstrom B, et al. Pharmacokinetics of P-glycoprotein inhibition in the rat blood-brain barrier. J Pharm Sci. 2008;97(12):5386–400.
Biliouris K, Gaitonde P, Yin W, Norris DA, Wang Y, Henry S, et al. A semi-mechanistic population pharmacokinetic model of Nusinersen: an antisense oligonucleotide for the treatment of spinal muscular atrophy. CPT Pharmacometrics Syst Pharmacol. 2018;7(9):581–92.
Westerhout J, Ploeger B, Smeets J, Danhof M, de Lange EC. Physiologically based pharmacokinetic modeling to investigate regional brain distribution kinetics in rats. AAPS J. 2012;14(3):543–53.
Westerhout J. Prediction of brain target site concentrations on the basis of CSF PK: Ipskamp Drukkers, Enschede; 2014.
Chiang MD, Frey K, Lee C, Kharasch ED, Tallchief D, Sawyer C, et al. Plasma and cerebrospinal fluid pharmacokinetics of ondansetron in humans. Br J Clin Pharmacol. 2020.
Geldof M, Freijer J, van Beijsterveldt L, Danhof M. Pharmacokinetic modeling of non-linear brain distribution of fluvoxamine in the rat. Pharm Res. 2008;25(4):792–804.
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported in part by a research grant (R01NS104500-01) from the National Institute of Neurological Disorders and Stroke (SH, LK), and by American Foundation for Pharmaceutical Education (AFPE) Pre-Doctoral Fellowship in Pharmaceutical Sciences (MC). The authors would like to thank Dr. John M. Harrold for development and support of the Ubiquity modeling framework.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chiang, M., Back, Hm., Lee, J.B. et al. Pharmacokinetic Modeling of the Impact of P-glycoprotein on Ondansetron Disposition in the Central Nervous System. Pharm Res 37, 205 (2020). https://doi.org/10.1007/s11095-020-02929-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02929-2