Abstract
Purpose
Nelfinavir (NFV), a FDA approved antiretroviral drug, has been reported to exhibit cancer cells growth inhibition and increased apoptosis. However, it requires a higher dose leading to toxicity, thus limiting its potential clinical translation. We aim to develop biodegradable (poly (lactic-co-glycolic acid)) PLGA nanoparticles of nelfinavir and determine their efficacy to treat non-small cell lung cancer (NSCLC).
Experimental Design
HIV protease inhibitor, NFV, was loaded into PLGA nanoparticles by double emulsion/solvent evaporation method; and nanoparticles were characterized for physicochemical characteristics including morphology and intracellular uptake. Their anti-cancer efficacy in NSCLC was assessed by in vitro assays including cytotoxicity, cellular migration, colony formation; and 3D spheroid culture mimicking in-vivo tumor microenvironment. Studies were also conducted to elucidate effects on molecular pathways including apoptosis, autophagy, and endoplasmic stress.
Results
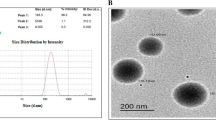
NFV loaded PLGA nanoparticles (NPs) were found to have particle size: 191.1 ± 10.0 nm, zeta potential: −24.3 ± 0.9 mV, % drug loading: 2.5 ± 0.0%; and entrapment efficiency (EE): 30.1 ± 0.5%. NFV NP inhibited proliferation of NSCLC cells compared to NFV and exhibited significant IC50 reduction. From the caspase-dependent apoptosis assays and western blot studies (upregulation of ATF3), it was revealed that NFV NP significantly induced ER stress marker ATF3, cleaved PARP and further caused autophagy inhibition (LC3BII upregulation) leading to increased cellular death. In addition, NFV NP were found to be more efficacious in penetrating solid tumors in ex-vivo studies compared to plain NFV.
Conclusions
Nelfinavir, a lead HIV protease inhibitor can be repositioned as a NSCLC therapeutic through nanoparticulate delivery. Given its ability to induce apoptosis and efficient tumor penetration capability, NFV loaded PLGA nanoparticulate systems provide a promising delivery system in NSCLC treatment.










Similar content being viewed by others
Abbreviations
- DAPI:
-
4′,6-diamidino-2-phenylindole
- DCM:
-
Dichloromethane
- %EE:
-
Encapsulation efficiency
- FBS:
-
Fetal bovine serum
- IC50 :
-
50% inhibition concentration
- ICH:
-
International council for harmonization of technical requirements for pharmaceuticals for human use
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- NFV:
-
Nelfinavir
- NFV NPs:
-
NFV loaded PLGA nanoparticles
- NSCLC:
-
Non-small-cell lung cancer
- PBS:
-
Phosphate-buffered saline
- PDI:
-
Polydispersity index
- PLGA:
-
Poly (lactic-co-glycolic acid)
- PS:
-
Particle size
- PVA:
-
Poly (vinyl alcohol)
- RH:
-
Relative humidity
- TEM:
-
Transmission electron microscopy
References
Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016 Jun;5(3):288–300.
Parvathaneni V, Kulkarni NS, Muth A, Gupta V. Drug repurposing: a promising tool to accelerate the drug discovery process. Drug Discov Today. 2019 Oct;24(10):2076–85.
Gong H, Liu F, Liu X, Min S, Wu N, Liu Xet al. APPBP2 enhances non-small cell lung cancer proliferation and invasiveness through regulating PPM1D and SPOP. EBioMedicine. 2019 May 16;44:138–149.
Yang Y, Ikezoe T, Nishioka C, Bandobashi K, Takeuchi T, Adachi Y, et al. NFV, an HIV-1 protease inhibitor, induces growth arrest, reduced Akt signalling, apoptosis and docetaxel sensitisation in NSCLC cell lines. Br J Cancer. 2006 Dec 18;95(12):1653–62.
Yadav RK, Chae S-W, Kim H-R, Chae HJ. Endoplasmic reticulum stress and cancer. J Cancer Prev. 2014 Jun;19(2):75–88.
Gills JJ, LoPiccolo J, Dennis PA. Nelfinavir, a new anti-cancer drug with pleiotropic effects and many paths to autophagy. Autophagy. 2008 Jan 1;4(1):107–9.
Liu G, Pei F, Yang F, Li L, Amin AD, Liu S, et al. Role of autophagy and apoptosis in non-small-cell lung cancer. Int J Mol Sci. 2017 Feb;10:18(2).
Chen Q, Kang J, Fu C. The independence of and associations among apoptosis, autophagy, and necrosis. Signal Transduct Target Ther. 2018 Jul 1;3(1):1–11.
Su M, Mei Y, Sinha S. Role of the crosstalk between autophagy and apoptosis in cancer. J Oncol. 2013;2013:e102735.
Torian L. HIV Surveillance --- United States, 1981--2008 [Internet]. 2011 Jun p. 689–93. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6021a2.htm
Srirangam A, Mitra R, Wang M, Gorski JC, Badve S, Baldridge L, et al. Effects of HIV protease inhibitor ritonavir on Akt-regulated cell proliferation in breast cancer. Clin Cancer Res. 2006 Mar 15;12(6):1883–96.
Jiang W, Mikochik PJ, Ra JH, Lei H, Flaherty KT, Winkler JD, et al. HIV protease inhibitor nelfinavir inhibits growth of human melanoma cells by induction of cell cycle arrest. Cancer Res. 2007 Feb 1;67(3):1221–7.
Maksimovic-Ivanic D, Fagone P, McCubrey J, Bendtzen K, Mijatovic S, Nicoletti F. HIV-protease inhibitors for the treatment of cancer: repositioning HIV protease inhibitors while developing more potent NO-hybridized derivatives? Int J Cancer. 2017;140(8):1713–26.
Rakashanda S, Amin S. Proteases as targets in anticancer therapy using their inhibitors. J Life Sci. 2013 Dec 1;5(2):133–8.
Trezza A, Cicaloni V, Pettini F, Spiga O. Chapter 2 - Potential roles of protease inhibitors in anticancer therapy. In: Gupta SP, editor. Cancer-Leading Proteases. Academic Press; 2020. p. 13–49.
Gills JJ, Lopiccolo J, Tsurutani J, Shoemaker RH, Best CJM, Abu-Asab MS, et al. Nelfinavir, a lead HIV protease inhibitor, is a broad-spectrum, anticancer agent that induces endoplasmic reticulum stress, autophagy, and apoptosis in vitro and in vivo. Clin Cancer Res Off J Am Assoc Cancer Res. 2007 Sep 1;13(17):5183–94.
Bernstein WB, Dennis PA. Repositioning HIV protease inhibitors as cancer therapeutics. Curr Opin HIV AIDS. 2008 Nov;3(6):666–75.
Xiang T, Du L, Pham P, Zhu B, Jiang S. Nelfinavir, an HIV protease inhibitor, induces apoptosis and cell cycle arrest in human cervical cancer cells via the ROS-dependent mitochondrial pathway. Cancer Lett. 2015 Aug 1;364(1):79–88.
Brüning A, Friese K, Burges A, Mylonas I. Tamoxifen enhances the cytotoxic effects of nelfinavir in breast cancer cells. Breast Cancer Res BCR. 2010;12(4):R45.
Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016 Mar;7(2):27–31.
Bryson YJ, Mirochnick M, Stek A, Mofenson LM, Connor J, Capparelli E, et al. Pharmacokinetics and safety of nelfinavir when used in combination with zidovudine and lamivudine in HIV-infected pregnant women: pediatric AIDS Clinical Trials Group (PACTG) protocol 353. HIV Clin Trials. 2008 Apr;9(2):115–25.
Panhard X, Goujard C, Legrand M, Taburet AM, Diquet B, Mentré F, et al. Population pharmacokinetic analysis for nelfinavir and its metabolite M8 in virologically controlled HIV-infected patients on HAART. Br J Clin Pharmacol. 2005 Oct;60(4):390–403.
Barry M, Mulcahy F, Merry C, Gibbons S, Back D. Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection. Clin Pharmacokinet. 1999 Apr;36(4):289–304.
Nelfinavir Mesylate in Treating Patients With Recurrent, Metastatic, or Unresectable Liposarcoma - Full Text View - ClinicalTrials.gov [Internet]. [cited 2020 Apr 2]. Available from: https://clinicaltrials.gov/ct2/show/NCT00233948
Suk KH, Gopinath SCB. Drug encapsulated nanoparticles for treating targeted cells. Curr Med Chem. 2017;24(30):3310–21.
Vaidya B, Kulkarni NS, Shukla SK, Parvathaneni V, Chauhan G, Damon JK, et al. Development of inhalable quinacrine loaded bovine serum albumin modified cationic nanoparticles: repurposing quinacrine for lung cancer therapeutics. Int J Pharm. 2020 Mar 15;577:118995.
Mendes LP, Sarisozen C, Luther E, Pan J, Torchilin VP. Surface-engineered polyethyleneimine-modified liposomes as novel carrier of siRNA and chemotherapeutics for combination treatment of drug-resistant cancers. Drug Deliv. 2019 Jan 1;26(1):443–58.
Majumder J, Taratula O, Minko T. Nanocarrier-based systems for targeted and site specific therapeutic delivery. Adv Drug Deliv Rev. 2019 Apr 1;144:57–77.
Lombardo D, Kiselev MA, Caccamo MT. Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. Fratoddi I, editor. J Nanomater. 2019 Feb 27;2019:3702518.
Martins JP. das Neves J, de la Fuente M, Celia C, Florindo H, Günday-Türeli N, et al. the solid progress of nanomedicine. Drug Deliv. Transl Res. 2020 Mar;5.
Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 2012 Dec 1;64:72–82.
Chandolu V, Dass CR. Treatment of lung cancer using nanoparticle drug delivery systems. Curr Drug Discov Technol. 2013 Jun;10(2):170–6.
Shukla SK, Kulkarni NS, Farrales P, Kanabar DD, Parvathaneni V, Kunda NK, et al. Sorafenib loaded inhalable polymeric Nanocarriers against non-small cell lung cancer. Pharm Res. 2020 Mar 12;37(3):67.
Vaidya B, Parvathaneni V, Kulkarni NS, Shukla SK, Damon JK, Sarode A, et al. Cyclodextrin modified erlotinib loaded PLGA nanoparticles for improved therapeutic efficacy against non-small cell lung cancer. Int J Biol Macromol. 2019 Feb 1;122:338–47.
Shukla SK, Kulkarni NS, Chan A, Parvathaneni V, Farrales P, Muth A, et al. Metformin-encapsulated liposome delivery system: an effective treatment approach against breast cancer. Pharmaceutics. 2019 Oct;28:11(11).
Gupta V, Gupta N, Shaik IH, Mehvar R, McMurtry IF, Oka M, et al. Liposomal Fasudil, a rho-kinase inhibitor, for prolonged pulmonary preferential vasodilation in pulmonary arterial hypertension. J Control Release Off J Control Release Soc. 2013 Apr 28;167(2):189–99.
Kulkarni NS, Parvathaneni V, Shukla SK, Barasa L, Perron JC, Yoganathan S, et al. Tyrosine kinase inhibitor conjugated quantum dots for non-small cell lung cancer (NSCLC) treatment. Eur J Pharm Sci. 2019 May 15;133:145–59.
Liang C-C, Park AY, Guan J-L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2(2):329–33.
Franken NAP, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1(5):2315–9.
Geissmann Q. OpenCFU, a new free and open-source software to count cell colonies and other circular objects. PLoS One. 2013 Feb 15;8(2):e54072.
Lazzari G, Couvreur P, Mura S. Multicellular tumor spheroids: a relevant 3D model for the in vitro preclinical investigation of polymer nanomedicines. Polym Chem. 2017 Aug 30;8(34):4947–69.
Tchoryk A, Taresco V, Argent RH, Ashford M, Gellert PR, Stolnik S, et al. Penetration and uptake of nanoparticles in 3D tumor spheroids. Bioconjug Chem. 2019 May 15;30(5):1371–84.
Lossi L, Castagna C, Merighi A. Caspase-3 mediated cell death in the Normal development of the mammalian cerebellum. Int J Mol Sci. 2018 Dec;12:19(12).
Gross K, Karagiannides I, Thomou T, Koon HW, Bowe C, Kim H, et al. Substance P promotes expansion of human mesenteric preadipocytes through proliferative and antiapoptotic pathways. Am J Physiol Gastrointest Liver Physiol. 2009 May;296(5):G1012–9.
Sithara T, Arun KB, Syama HP, Reshmitha TR, Nisha P. Morin inhibits proliferation of SW480 colorectal cancer cells by inducing apoptosis mediated by reactive oxygen species formation and uncoupling of Warburg effect. Front Pharmacol. 2017;8:640.
Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, et al. Impact of particle size and polydispersity index on the clinical applications of Lipidic Nanocarrier systems. Pharmaceutics. 2018 May;18:10(2).
Perry JL, Reuter KG, Luft JC, Pecot CV, Zamboni W, DeSimone JM. Mediating passive tumor accumulation through particle size, tumor type, and location. Nano Lett. 2017 May 10;17(5):2879–86.
Dwivedi R, Singh AK, Dhillon A. pH-responsive drug release from dependal-M loaded polyacrylamide hydrogels. J Sci Adv Mater Devices. 2017 Mar 1;2(1):45–50.
Eaton P, Quaresma P, Soares C, Neves C, de Almeida MP, Pereira E, et al. A direct comparison of experimental methods to measure dimensions of synthetic nanoparticles. Ultramicroscopy. 2017 Nov 1;182:179–90.
Muthu MS, Feng S-S. Pharmaceutical stability aspects of nanomedicines. Nanomed. 2009 Dec 1;4(8):857–60.
Santamaría-Aguirre J, Alcocer-Vallejo R, López-Fanárraga M. Drug nanoparticle stability assessment using isothermal and nonisothermal approaches. J Nanomater. 2018;2018:e3047178.
Zhao Y-Z, Sun C-Z, Lu C-T, Dai D-D, Lv H-F, Wu Y, et al. Characterization and anti-tumor activity of chemical conjugation of doxorubicin in polymeric micelles (DOX-P) in vitro. Cancer Lett. 2011;311(2):187–94.
Oh JE, Nam YS, Lee KH, Park TG. Conjugation of drug to poly (D, L-lactic-co-glycolic acid) for controlled release from biodegradable microspheres. J Control Release. 1999;57(3):269–80.
Soprano M, Sorriento D, Rusciano MR, Maione AS, Limite G, Forestieri P, et al. Oxidative stress mediates the Antiproliferative effects of nelfinavir in breast cancer cells. PLoS One. 2016 Jun;9:11(6).
Dunlop EA, Johnson CE, Wiltshire M, Errington RJ, Tee AR. Targeting protein homeostasis with nelfinavir/salinomycin dual therapy effectively induces death of mTORC1 hyperactive cells. Oncotarget. 2017 Jul 25;8(30):48711–24.
Brüning A, Rahmeh M, Gingelmaier A, Friese K. The mitochondria-independent cytotoxic effect of nelfinavir on leukemia cells can be enhanced by sorafenib-mediated mcl-1 downregulation and mitochondrial membrane destabilization. Mol Cancer. 2010 Jan 27;9:19.
Koltai T. Nelfinavir and other protease inhibitors in cancer: mechanisms involved in anticancer activity. F1000Research. 2015;4:9.
Rajendran V, Jain MV. In vitro tumorigenic assay: Colony forming assay for cancer stem cells. Methods Mol Biol Clifton NJ. 1692;2018:89–95.
Parvathaneni V, Kulkarni NS, Shukla SK, Farrales PT, Kunda NK, Muth A, et al. Systematic development and optimization of inhalable Pirfenidone liposomes for non-small cell lung cancer treatment. Pharmaceutics. 2020 Feb;28:12(3).
Breslin S, O’Driscoll L. Three-dimensional cell culture: the missing link in drug discovery. Drug Discov Today. 2013 Mar;18(5–6):240–9.
Fennema E, Rivron N, Rouwkema J, van Blitterswijk C, de Boer J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013 Feb;31(2):108–15.
Cullen SP, Martin SJ. Caspase activation pathways: some recent progress. Cell Death Differ. 2009 Jul;16(7):935–8.
Ferraro-Peyret C, Quemeneur L, Flacher M, Revillard J-P, Genestier L. Caspase-independent phosphatidylserine exposure during apoptosis of primary T lymphocytes. J Immunol. 2002 Nov 1;169(9):4805–10.
Parker RA, Flint OP, Mulvey R, Elosua C, Wang F, Fenderson W, et al. Endoplasmic reticulum stress links dyslipidemia to inhibition of proteasome activity and glucose transport by HIV protease inhibitors. Mol Pharmacol. 2005 Jun;67(6):1909–19.
Mimnaugh EG, Xu W, Vos M, Yuan X, Neckers L. Endoplasmic reticulum vacuolization and valosin-containing protein relocalization result from simultaneous hsp90 inhibition by geldanamycin and proteasome inhibition by velcade. Mol Cancer Res MCR. 2006 Sep;4(9):667–81.
Armando RG, Mengual Gómez DL, Gomez DE. New drugs are not enough-drug repositioning in oncology: an update. Int J Oncol. 2020 Mar 1;56(3):651–84.
Rengan R, Mick R, Pryma DA, Lin LL, Christodouleas J, Plastaras JP, et al. Clinical outcomes of the HIV protease inhibitor nelfinavir with concurrent Chemoradiotherapy for Unresectable stage IIIA/IIIB non-small cell lung cancer: a phase 1/2 trial. JAMA Oncol. 2019 Aug;22.
Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres M d P, Acosta-Torres LS, et al. Nano based drug delivery systems: recent developments and future prospects J Nanobiotechnology. 2018 Sep;19:16.
Khan MN, Haggag YA, Lane ME, McCarron PA, Tambuwala MM. Polymeric Nano-encapsulation of curcumin enhances its anti-cancer activity in breast (MDA-MB231) and lung (A549) cancer cells through reduction in expression of HIF-1α and nuclear p65 (Rel a). Curr Drug Deliv. 2018 Feb 14;15(2):286–95.
Venkatesh DN, Baskaran M, Karri VVSR, Mannemala SS, Radhakrishna K, Goti S. Fabrication and in vivo evaluation of nelfinavir loaded PLGA nanoparticles for enhancing oral bioavailability and therapeutic effect. Saudi Pharm J SPJ. 2015 Nov;23(6):667–74.
Hotze EM, Phenrat T, Lowry GV. Nanoparticle aggregation: challenges to understanding transport and reactivity in the environment. J Environ Qual. 2010;39(6):1909–24.
Müller KH, Motskin M, Philpott AJ, Routh AF, Shanahan CM, Duer MJ, et al. The effect of particle agglomeration on the formation of a surface-connected compartment induced by hydroxyapatite nanoparticles in human monocyte-derived macrophages. Biomaterials. 2014 Jan 1;35(3):1074–88.
Li C, Zhao L, Han J, Wang R, Xiong C, Xie X. Synthesis of citrate-stabilized hydrocolloids of hydroxyapatite through a novel two-stage method: a possible aggregates–breakdown mechanism of colloid formation. J Colloid Interface Sci. 2011 Aug 15;360(2):341–9.
Baishya H. Application of Mathematical Models in Drug Release Kinetics of Carbidopa and Levodopa ER Tablets. J Dev Drugs. 2017;06(02).
Bruschi ML, editor. 5 - Mathematical models of drug release. Woodhead Publishing; 2015.
Behzadi S, Serpooshan V, Tao W, Hamaly MA, Alkawareek MY, Dreaden EC, et al. Cellular uptake of nanoparticles: journey inside the cell. Chem Soc Rev. 2017 Jul 17;46(14):4218–44.
Hulkower KI, Herber RL. Cell migration and invasion assays as tools for drug discovery. Pharmaceutics. 2011 Mar 11;3(1):107–24.
Justus CR, Leffler N, Ruiz-Echevarria M, Yang LV. In vitro cell migration and invasion assays. J Vis Exp JoVE. 2014 Jun 1;88.
Wang X, Decker CC, Zechner L, Krstin S, Wink M. In vitro wound healing of tumor cells: inhibition of cell migration by selected cytotoxic alkaloids. BMC Pharmacol Toxicol. 2019 Jan 9;20(1):4.
Pijuan J, Barceló C, Moreno DF, Maiques O, Sisó P, Marti RM, et al. In vitro cell migration, invasion, and adhesion assays: from cell imaging to data analysis. Front Cell Dev Biol. 2019;7.
Amaral RLF, Miranda M, Marcato PD, Swiech K. Comparative analysis of 3D bladder tumor spheroids obtained by forced floating and hanging drop methods for drug screening. Front Physiol. 2017 Aug;22:8.
Bresciani G, Hofland LJ, Dogan F, Giamas G, Gagliano T, Zatelli MC. Evaluation of spheroid 3D culture methods to study a pancreatic neuroendocrine neoplasm cell line. Front Endocrinol. 2019;10.
Parrish AB, Freel CD, Kornbluth S. Cellular Mechanisms Controlling Caspase Activation and Function. Cold Spring Harb Perspect Biol. 2013 Jun;5(6).
Balaji N, Devy AS, Sumathi MK, Vidyalakshmi S, Kumar GS, D’Silva S. Annexin V – affinity assay – apoptosis detection system in granular cell Ameloblastoma. J Int Oral Health JIOH. 2013 Dec;5(6):25–30.
Ap D. Beyond annexin V: fluorescence response of cellular membranes to apoptosis. Cytotechnology. 2012 Jul 14;65(2):157–72.
Lin Y, Jiang M, Chen W, Zhao T, Wei Y. Cancer and ER stress: mutual crosstalk between autophagy, oxidative stress and inflammatory response. Biomed Pharmacother. 2019 Oct 1;118:109249.
O’brien A, Barber JEB, Reid S, Niknejad N, Dimitroulakos J. Enhancement of cisplatin cytotoxicity by disulfiram involves activating transcription factor 3. Anticancer Res. 2012 Jul 1;32(7):2679–88.
Redmann M, Benavides GA, Berryhill TF, Wani WY, Ouyang X, Johnson MS, et al. Inhibition of autophagy with bafilomycin and chloroquine decreases mitochondrial quality and bioenergetic function in primary neurons. Redox Biol. 2016 Nov 18;11:73–81.
Lim S-J, Choi MK, Kim MJ, Kim JK. Alpha-tocopheryl succinate potentiates the paclitaxel-induced apoptosis through enforced caspase 8 activation in human H460 lung cancer cells. Exp Mol Med. 2009 Oct 31;41(10):737–45.
Acknowledgments
The author(s) would like to acknowledge the Imaging Facility of CUNY Advanced Science Research Center for instrument use, scientific and technical assistance; and Dr. Aaron Muth, Assistant Professor at St. John’s university for providing EVOS FL fluorescence microscope. The author(s) would also like to acknowledge Dr. Yong Yu (Associate Professor & Graduate Director, Biological Sciences, St. John’s University, NY, USA) for providing porcine kidney proximal tubule cell line (LLC-PK1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial Disclosure and Conflict of Interest
This study was supported by start-up funds to VG from College of Pharmacy and Health Sciences (CPHS), St. John’s University. VP was supported by teaching assistantships from CPHS. MG was supported by graduate assistantship from University Learning Commons (ULC) at St. John’s University. SKS and NSK were supported with the research assistantship by National Institutes of Health (NIH) (Grant #1R15HL138606-01A1) to Vivek Gupta.
All authors disclose no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 976 kb)
Rights and permissions
About this article
Cite this article
Parvathaneni, V., Goyal, M., Kulkarni, N.S. et al. Nanotechnology Based Repositioning of an Anti-Viral Drug for Non-Small Cell Lung Cancer (NSCLC). Pharm Res 37, 123 (2020). https://doi.org/10.1007/s11095-020-02848-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02848-2