Abstract
Purpose
The purpose of this study was (a) to suggest a novel dermatopharmacokinetic (DPK) approach from which pharmacokinetic parameters relevant to the bioequivalence (BE) assessment of a topical formulation can be deduced while circumventing the need for numerous measurements and assumptions, and (b) to investigate whether this approach enables the correct conclusion of BE and bioinequivalence (BIE).
Methods
Bioequivalent and bioinequivalent formulations of acyclovir were compared versus a reference product (Zovirax®). Tape Stripping was conducted at only one dose duration during the uptake phase to generate drug content in stratum corneum versus time profiles, each time point corresponding to one stripped layer. Nonlinear mixed effect modeling (ADAPT5®) (MLEM algorithm) was used to fit the DPK data and to estimate the rate (Kin) and extent (FS) of drug absorption/input into the skin. Results were evaluated using the average BE approach.
Results
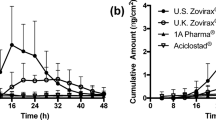
Estimated exposure metrics were within the usual BE limits for the bioequivalent formulation (FS: 102.4 [90%CI: 97.5–107.7]; Kin: 94.2 [90%CI: 83.7–106.0]), but outside those limits for the bioinequivalent formulation (FS: 43.4 [90%CI: 27.9–67.6]; Kin: 54.5 [90%CI: 36.6–81.1]).
Conclusions
The proposed novel DPK approach was shown to be successful, robust and applicable to assess BE and BIE correctly between topical formulations.




Similar content being viewed by others
Notes
Studied ACV creams are locally effective topical formulations; therefore, input into the skin should be preferred over absorption which is rather reflective of systemic exposure. For the purpose of simplicity, however, absorption and input into the skin may interchangeably be used throughout this text.
Abbreviations
- ACV:
-
Acyclovir
- API:
-
Active pharmaceutical ingredient
- AUC:
-
Area Under Curve
- BA:
-
Bioavailability
- BE:
-
Bioequivalence/Bioequivalent
- BIE:
-
Bioinequivalence/Bioinequivalent
- Cmax :
-
Maximum concentration
- CPT:
-
Compartment
- CV:
-
Coefficient of variation
- DD:
-
Dose Duration
- DPK:
-
Dermatopharmacokinetics
- ED 50 :
-
The dose duration at which 50% of Emax is obtained
- E max :
-
Maximum effect
- FDA:
-
Food and Drug Administration
- FS :
-
Extent of absorption/input into the skin
- GMR:
-
Geometric Mean Ratio
- GOF:
-
Goodness-of-Fit
- HV:
-
Healthy volunteers
- IOV:
-
Inter-Occasion Variability
- IPRED:
-
Individual-level predictions
- Kdiff :
-
Diffusion rate constant
- Kin :
-
First-order absorption/input rate constant into the skin
- Kout :
-
Diffusion rate constant out from the last compartment
- Max:
-
Maximum
- Min:
-
Minimum
- ML:
-
Maximum Likelihood
- MLEM:
-
Maximum Likelihood Expectation Maximization
- MOF:
-
Minimum Value of the Objective Function
- PK:
-
Pharmacokinetics
- Pop:
-
Population
- PRED:
-
Population-level predictions
- Qmax :
-
Maximum amount
- Ref:
-
Reference
- RLD:
-
Reference Listed Drug
- RP:
-
Reference Product
- SC:
-
Stratum Corneum
- STS:
-
Standard 2-Stage
- TOST:
-
Two One-Sided Tests
- TS:
-
Tape Stripping
- VPC:
-
Visual Predictive Check
- WRES:
-
Weighted residuals
References
Chen ML, Lesko L, Williams RL. Measures of exposure versus measures of rate and extent of absorption. Clin Pharmacokinet. 2001;40(8):565–72.
Herkenne C, Alberti I, Naik A, Kalia YN, Mathy F-X, Préat V, et al. In vivo methods for the assessment of topical drug bioavailability. Pharm Res. 2007;25(1):87.
Alberti I, Kalia YN, Naik A, Guy RH. Assessment and prediction of the cutaneous bioavailability of topical Terbinafine, in vivo, in man. Pharm Res. 2001;18(10):1472–5.
Borsadia S, Ghanem AH, Seta Y, Higuchi WI, Flynn GL, Behl CR, et al. Factors to be considered in the evaluation of bioavailability and bioequivalence of topical formulations. Skin Pharmacol. 1992;5(3):129–45.
Yacobi A, Shah VP, Bashaw ED, Benfeldt E, Davit B, Ganes D, et al. Current challenges in bioequivalence, quality, and novel assessment technologies for topical products. Pharm Res. 2014;31(4):837–46.
Shah VP, Flynn GL, Yacobi A, Maibach HI, Bon C, Fleischer NM, et al. Bioequivalence of topical dermatological dosage forms-methods of evaluation of bioequivalence. Pharm Res. 1998;15(2):167–71.
United States Food and Drug Administration, Title 21 Code of Federal Regulations (CFR) Part 320, Section 24. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=320.24. Accessed 8 Feb 2018.
Braddy AC, Davit BM, Stier EM, Conner DP. Survey of international regulatory bioequivalence recommendations for approval of generic topical dermatological drug products. AAPS J. 2015;17(1):121–33.
Rougier A, Dupuis D, Lotte C, Roguet R, Wester RC, Maibach HI. Regional variation in percutaneous absorption in man: measurement by the stripping method. Arch Dermatol Res. 1986;278(6):465–9.
Rougier A, Dupuis D, Lotte C, Roguet R, Schaefer H. In vivo correlation between stratum corneum reservoir function and percutaneous absorption. J Invest Dermatol. 1983;81(3):275–8.
Rougier A, Rallis M, Krien P, Lotte C. In vivo percutaneous absorption: a key role for stratum corneum/vehicle partitioning. Arch Dermatol Res. 1990;282(8):498–505.
Rougier A, Lotte C, Maibach HI. In vivo percutaneous penetration of some organic compounds related to anatomic site in humans: predictive assessment by the stripping method. J Pharm Sci. 1987;76(6):451–4.
Raney SG, Franz TJ, Lehman PA, Lionberger R, Chen M-L. Pharmacokinetics-based approaches for bioequivalence evaluation of topical dermatological drug products. Clin Pharmacokinet. 2015;54(11):1095–106.
Herkenne C, Naik A, Kalia YN, Hadgraft J, Guy RH. Dermatopharmacokinetic prediction of topical drug bioavailability in vivo. J Invest Dermatol. 2007;127(4):887–94.
Pershing LK, Bakhtian S, Poncelet CE, Corlett JL, Shah VP. Comparison of skin stripping, in vitro release, and skin blanching response methods to measure dose response and similarity of triamcinolone acetonide cream strengths from two manufactured sources. J Pharm Sci. 2002;91(5):1312–23.
US-FDA Guidance for Industry: Topical dermatologic drug product NDAs and ANDAs-in vivo bioavailability, bioequivalence, in vitro release, and associated studies, 1998. Bethesda (MD): Center for Drug Evaluation and Research, FDA.
Franz TJ. Study #1, Avita gel 0.025% vs Retin-A gel 0.025%. Transcribed presentation to the Advisory Committee for Pharmaceutical Sciences Meeting, Center for Drug Evaluation and Research (CDER), Food and Drug Administration (FDA), Rockville, MD, November 29, 2001. Transcript of presentation. pp. 47–61. http://www.fda.gov/ohrms/dockets/ac/01/transcripts/3804t2_01_Morning_Session.pdf. Accessed 8 Feb 2018.
Pershing LK, Nelson JL, Corlett JL, Shrivastava SP, Hare DB, Shah VP. Assessment of dermatopharmacokinetic approach in the bioequivalence determination of topical tretinoin gel products. J Am Acad Dermatol. 2003;48(5):740–51.
Kalia YN, Alberti I, Sekkat N, Curdy C, Naik A, Guy RH. Normalization of stratum corneum barrier function and transepidermal water loss in vivo. Pharm Res. 2000;17(9):1148–50.
Pirot F, Berardesca E, Kalia YN, Singh M, Maibach HI, Guy RH. Stratum corneum thickness and apparent water diffusivity: facile and noninvasive quantitation in vivo. Pharm Res. 1998;15(3):492–4.
Alberti I, Kalia YN, Naik A, Bonny J, Guy RH. Effect of ethanol and isopropyl myristate on the availability of topical terbinafine in human stratum corneum, in vivo. Int J Pharm. 2001;219(1–2):11–9.
Pirot F, Kalia YN, Stinchcomb AL, Keating G, Bunge A, Guy RH. Characterization of the permeability barrier of human skin in vivo. Proc Natl Acad Sci U S A. 1997;94(4):1562–7.
Cordery SF, Pensado A, Chiu WS, Shehab MZ, Bunge AL, Delgado-Charro MB, et al. Topical bioavailability of diclofenac from locally-acting, dermatological formulations. Int J Pharm. 2017;529(1–2):55–64.
N’Dri-Stempfer B, Navidi WC, Guy RH, Bunge AL. Improved bioequivalence assessment of topical dermatological drug products using Dermatopharmacokinetics. Pharm Res. 2008;26(2):316.
N'Dri-Stempfer B, Navidi WC, Guy RH, Bunge AL. Optimizing metrics for the assessment of bioequivalence between topical drug products. Pharm Res. 2009;25(7):1621–30.
Navidi W, Hutchinson A, N’Dri-Stempfer B, Bunge A. Determining bioequivalence of topical dermatological drug products by tape-stripping. J Pharmacokinet Pharmacodyn. 2008;35(3):337.
Bunge A, N’Dri-Stempfer B, Navidi W, Guy R. Final report to food and drug administration (FDA): therapeutic equivalence of topical products, Colorado School of Mines, Golden, Colorado. 2007.
Nallagundla S, Patnala S, Kanfer I. Application of an optimized tape stripping method for the bioequivalence assessment of topical acyclovir creams. AAPS PharmSciTech. 2018;19(4):1567–73.
Parfitt NR, Skinner MF, Bon C, Kanfer I. Bioequivalence of topical clotrimazole formulations: an improved tape stripping method. J Pharm Pharm Sci. 2011;14(3):347–57.
Au WL, Skinner M, Kanfer I. Comparison of tape stripping with the human skin blanching assay for the bioequivalence assessment of topical clobetasol propionate formulations. J Pharm Pharm Sci. 2010;13(1):11–20.
Riku J, Muranushi N. Japan. In: Kanfer I, editor. Bioequivalence Requirements in Various Global Jurisdictions. Cham: Springer International Publishing; 2017. p. 127–58.
Kanfer I. Methods for the assessment of bioequivalence of topical dosage forms: correlations, optimization strategies, and innovative approaches. In: Shah VP, Maibach HI, Jenner J, editors. Topical drug bioavailability, bioequivalence, and penetration. New York: Springer New York; 2014. p. 113–51.
Kanfer I, Tettey-Amlalo RNO, Au WL, Hughes-Formella B. Assessment of topical dosage forms intended for local or regional activity. In: Shargel L, Kanfer I, editors. Generic drug product development specialty dosage forms. New York: Informa Healthcare USA; 2010. p. 54–103.
D'Argenio DZ, Schumitzky A, Wang X. ADAPT 5 User’s guide: pharmacokinetic/Pharmacodynamic systems analysis software. Los Angeles: Biomedical Simulations Resource; 2009.
Schuirmann DJ. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm. 1987;15(6):657–80.
FDA Draft Guidance on Warfarin Sodium. Guidelines, bioequivalence recommendation for specific products. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm201283.pdf. Accessed 22 May 2018.
Russell LM, Guy RH. Measurement and prediction of the rate and extent of drug delivery into and through the skin. Expert Opin Drug Deliv. 2009;6(4):355–69.
Olsen EA. A double-blind controlled comparison of generic and trade-name topical steroids using the vasoconstriction assay. Arch Dermatol. 1991;127(2):197–201.
Pinkus H. Examination of the epidermis by the strip method of removing horny layers. I. Observations on thickness of the horny layer, and on mitotic activity after stripping. J Invest Dermatol. 1951;16(6):383–6.
Duan JZ. Pharmacokinetics of Oral absorption. In: Shargel L, Yu ABC, editors. Applied Biopharmaceutics & Pharmacokinetics; pharmacokinetics, 7e. New York: McGraw-Hill Education; 2016.
Scheuplein RJ, Blank IH. Permeability of the skin. Physiol Rev. 1971;51(4):702–47.
Elias PM. Epidermal lipids, membranes, and keratinization. Int J Dermatol. 1981;20(1):1–19.
Kalia YN, Pirot F, Guy RH. Homogeneous transport in a heterogeneous membrane: water diffusion across human stratum corneum in vivo. Biophys J. 1996;71(5):2692–700.
Reddy MB, Stinchcomb AL, Guy RH, Bunge AL. Determining dermal absorption parameters in vivo from tape strip data. Pharm Res. 2002;19(3):292–8.
de Araujo TP, Fittipaldi IM, Bedor DCG, Duarte ML, Cordery SF, Guy RH, et al. Topical bio(in)equivalence of metronidazole formulations in vivo. Int J Pharm. 2018;541(1–2):167–72.
Crank J. Diffusion in a plane sheet. The mathematics of diffusion. London: Oxford University Press; 1975. p. 44–68.
Tsai JC, Cheng CL, Tsai YF, Sheu HM, Chou CH. Evaluation of in vivo bioequivalence methodology for topical clobetasol 17-propionate based on pharmacodynamic modeling using Chinese skin. J Pharm Sci. 2004;93(1):207–17.
Fradette C, Lavigne J, Waters D, Ducharme MP. The utility of the population approach applied to bioequivalence in patients: comparison of 2 formulations of cyclosporine. Ther Drug Monit. 2005;27(5):592–600.
Panhard X, Mentre F. Evaluation by simulation of tests based on non-linear mixed-effects models in pharmacokinetic interaction and bioequivalence cross-over trials. Stat Med. 2005;24(10):1509–24.
Alberti I, Kalia YN, Naik A, Bonny J-D, Guy RH. In vivo assessment of enhanced topical delivery of terbinafine to human stratum corneum. J Control Release. 2001;71(3):319–27.
Herkenne C, Naik A, Kalia YN, Hadgraft J, Guy RH. Pig ear skin ex vivo as a model for in vivo Dermatopharmacokinetic studies in man. Pharm Res. 2006;23(8):1850–6.
Wiedersberg S, Naik A, Leopold CS, Guy RH. Pharmacodynamics and dermatopharmacokinetics of betamethasone 17-valerate: assessment of topical bioavailability. Br J Dermatol. 2009;160(3):676–86.
Herkenne C, Naik A, Kalia YN, Hadgraft J, Guy RH. Effect of propylene glycol on ibuprofen absorption into human skin in vivo. J Pharm Sci. 2008;97(1):185–97.
Herkenne C, Naik A, Kalia YN, Hadgraft J, Guy RH. Ibuprofen transport into and through skin from topical formulations: in vitro-in vivo comparison. J Invest Dermatol. 2007;127(1):135–42.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ozdin, D., Kanfer, I. & Ducharme, M.P. Novel Approach for the Bioequivalence Assessment of Topical Cream Formulations: Model-Based Analysis of Tape Stripping Data Correctly Concludes BE and BIE. Pharm Res 37, 20 (2020). https://doi.org/10.1007/s11095-019-2724-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-019-2724-2