Abstract
Purpose
Oral direct compressible tablets are the most frequently used drug products. Manufacturing of tablets requires design and development of formulations, which need a number of excipients. The choice of excipients depends on the concentration, manufacturability, stability, and bioavailability of the active pharmaceutical ingredients (APIs). At MIT, we developed a miniature platform for on-demand manufacturing of direct compressible tablets. This study investigated how formulations could be simplified to use a small number of excipients for a number of different API’s in which long term stability is not required.
Method
Direct compressible tablets of five pharmaceutical drugs, Diazepam, Diphenhydramine HCl, Doxycycline Monohydrate, Ibuprofen, and Ciprofloxacin HCl, with different drug loadings, were made using direct compression in an automated small scale system.. The critical quality attributes (CQA) of the tablets were assessed for the quality standards set by the United States Pharmacopeia (USP).
Results
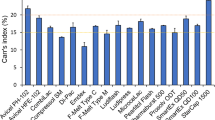
This miniature system can manufacture tablets - on-demand from crystalline API using the minimum number of excipients required for drug product performance. All drug tablets met USP quality standards after manufacturing and after 2 weeks of accelerated stability test, except for slightly lower drug release for Ibuprofen.
Conclusions
On-demand tablets manufacturing where there is no need for long term stability using a flexible, miniature, automated (integrated) system will simplify pharmaceutical formulation design compared to traditional formulations. This advancement will offer substantial economic benefits by decreasing product time-to-market and enhancing quality.




Similar content being viewed by others
Abbreviations
- API:
-
Active pharmaceutical ingredient
- AV:
-
Acceptance value
- BCS:
-
Biopharmaceutics classification system
- CIPRO:
-
Ciprofloxacin HCl
- CQA:
-
Critical quality attributes
- DARPA:
-
Defense Advanced Research Project Agency
- DC:
-
Direct compression
- DIA:
-
Diazepam
- DPH:
-
Diphenhydramine HCl
- DOX:
-
Doxycycline monohydrate
- FT:
-
Freeman technology
- ffc:
-
Flow function coefficient
- GIT:
-
Gastrointestinal tract
- GMP:
-
Good manufacturing practice
- HPLC:
-
High performance liquid chromatography
- IBU:
-
Ibuprofen
- MCC:
-
Microcrystalline cellulose
- MIT:
-
Massachusetts Institute of Technology
- NF:
-
National formulary
- PAT:
-
Process analytical technologies
- QbD:
-
Quality-by-design
- Q value:
-
Percentage of label claim of drug dissolved
- RH:
-
Relative humidity
- RSD:
-
Relative standard deviation
- SMCC:
-
Silicified microcrystalline cellulose
- t80 :
-
Time it takes for 80% of the drug to dissolve
- USP:
-
United States Pharmacopeia
- UV:
-
Ultraviolet
References
Darji MA, Lalge RM, Marathe SP, Mulay TD, Fatima T, Alshammari A, et al. Excipient stability in oral solid dosage forms: a review. AAPS PharmSciTech. 2018;19:12–26.
Augsburgerand LL, Hoag SW. Pharmaceutical dosage forms-tablets. CRC Press; 2016.
Qiu Y, Chen Y, Zhang GG, Yu L, Mantri RV. Developing solid oral dosage forms: pharmaceutical theory and practice. Academic Press; 2016.
Murthyand SN, Repka MA. Excipient stability: a critical aspect in stability of pharmaceuticals. Springer; 2017.
Gad SC. Oral drug formulation development in pharmaceutical lead selection stage. Oral formulation roadmap from early drug discovery to development. 2017. p. 339.
Lawrence XY, Amidon G, Khan MA, Hoag SW, Polli J, Raju G, et al. Understanding pharmaceutical quality by design. AAPS J. 2014;16:771–83.
Narang AS, Desai D, Badawy S. Impact of excipient interactions on solid dosage form stability. Excipient applications in formulation design and drug delivery. Springer; 2015, pp. 93–137.
Meier R, Moll KP, Krumme M, Kleinebudde P. Simplified, high druge-loaded formulations containing hydrochlorothiazide for twin-screw granulation. Chem Ing Tech. 2017;89:1025–33.
Meier R, Thommes M, Rasenack N, Krumme M, Moll K-P, Kleinebudde P. Simplified formulations with high drug loads for continuous twin-screw granulation. Int J Pharm. 2015;496:12–23.
Azad MA, Osorio JG, Brancazio D, Hammersmith G, Klee DM, Rapp K, et al. A compact, portable, re-configurable, and automated system for on-demand pharmaceutical tablet manufacturing. Int J Pharm. 2018;539:157–64.
A.P. Review. SuperTab®21AN Anhydrous lactose from DFE Pharma. https://www.americanpharmaceuticalreview.com/25260-Excipients/10570382-SuperTab-21AN-Anhydrous-lactose/. Accessed 28 May 2019.
D. Pharma. SuperTab® 21AN. https://www.dfepharma.com/excipients/oral-solid-dose/lactose/anhydrous/supertab-21an Accessed 28 May 2019.
Amidon GE, Secreast PJ, Mudie D. Particle, powder, and compact characterization. Developing solid oral dosage forms: Pharmaceutical theory and practice: Academic Press; New York, 2009. p. 163–86.
Knieke C, Azad MA, D. To, Bilgili E, Davé RN. Sub-100 micron fast dissolving nanocomposite drug powders. Powder Technol. 2015;271:49–60.
Schulze D. Powders and bulk solids. Behaviour, characterization, storage and flow. Springer; 2008. pp. 35–74.
Freeman R. Measuring the flow properties of consolidated, conditioned and aerated powders—a comparative study using a powder rheometer and a rotational shear cell. Powder Technol. 2007;174:25–33.
DailyMed. Current medication information-Diazepam 10 mg tablet, Diphenhydramine HCl 25 mg tablet, Doxycycline Monohydrate 50 mg tablet, Ibuprofen 200 mg tablet, Ciprofloxacin HCl 250 mg tablet. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=554baee5-b171-4452-a50a-41a0946f956c; https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=702f9786-7ce9-43e4-921d-e1db09612127;https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bf667704-f439-4e8a-a4a5-dd150462a125; https://dailymed.nlm.nih.gov/dailymed/search.cfm?labeltype=all&query=IBUPROFEN+200+MG; https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2e4adfff-e6d5-4031-903d-16eaf4bf74d7. Accessed 22 Sept 2019.
Rowe RC, Sheskey PJ, Quinn M. Handbook of pharmaceutical excipients–7th edition. Pharm Dev Technol. 2013;18:544.
Razavi SM, Callegari G, Drazer G, Cuitiño AM. Toward predicting tensile strength of pharmaceutical tablets by ultrasound measurement in continuous manufacturing. Int J Pharm. 2016;507:83–9.
Pharmacopeia, U. S. “USP 39 NF 34”. 2015.
Pharmacopeia, U. S. “USP–NF General Chapter<905> Uniformity of Dosage Units”. 2015.
Newton J, Rowley G, Fell J, Peacock D, Ridgway K. Computer analysis of the relation between tablet strength and compaction pressure. J Pharm Pharmacol. 23:1971.
Abdullahand E, Geldart D. The use of bulk density measurements as flowability indicators. Powder Technol. 1999;102:151–65.
Sun CC. Setting the bar for powder flow properties in successful high speed tableting. Powder Technol. 2010;201:106–8.
Han X, Ghoroi C, Davé R. Dry coating of micronized API powders for improved dissolution of directly compacted tablets with high drug loading. Int J Pharm. 2013;442:74–85.
Jenike AW. Storage and flow of solids. Bulletin No. 123; Vol. 53, No. 26, November 1964, Utah Univ., Salt Lake City (USA) 1976.
Seton L, Roberts M, Ur-Rehman F. Compaction of recrystallised ibuprofen. Chem Eng J. 2010;164:449–52.
Tye CK, Sun CC, Amidon GE. Evaluation of the effects of tableting speed on the relationships between compaction pressure, tablet tensile strength, and tablet solid fraction. J Pharm Sci. 2005;94:465–72.
Sinka I, Motazedian F, Cocks A, Pitt K. The effect of processing parameters on pharmaceutical tablet properties. Powder Technol. 2009;189:276–84.
Sun W-J, Aburub A, Sun CC. Particle engineering for enabling a formulation platform suitable for manufacturing low-dose tablets by direct compression. J Pharm Sci. 2017.
Nokhodchiand A, Javadzadeh Y. The effect of storage conditions on the physical stability of tablets. Pharm Technol Eur. 2007;19:20.
Alderbornand G, Ahlneck C. Moisture adsorption and tabletting. III Effect on tablet strenght-post compaction storage time profiles. Int J Pharm. 1991;73:249–58.
Cory WC, Harris C, Martinez S. Accelerated degradation of ibuprofen in tablets. Pharm Dev Technol. 2010;15:636–43.
Narang AS, Rao VM, Raghavan KS. Excipient compatibility. Developing solid oral dosage forms. Elsevier; 2009. pp. 125–145.
Saleki-Gerhardt A, Ahlneck C, Zografi G. Assessment of disorder in crystalline solids. Int J Pharm. 1994;101:237–47.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 41.3 kb)
Rights and permissions
About this article
Cite this article
Azad, M.A., Osorio, J.G., Wang, A. et al. On-Demand Manufacturing of Direct Compressible Tablets: Can Formulation Be Simplified?. Pharm Res 36, 167 (2019). https://doi.org/10.1007/s11095-019-2716-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-019-2716-2