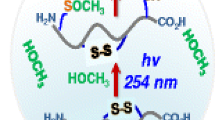
Abstract
Purpose
Thiol-disulfide exchange was monitored in recombinant human growth hormone (hGH) and in model tryptic peptides derived from hGH to investigate the effects of higher-order structure on the reaction.
Methods
Different free thiol-containing peptides, varying in length and amino acid sequence, were used to initiate the reaction at pH 7.0 and 37°C in hGH. Protein samples were digested with trypsin and analyzed for native disulfides, scrambled disulfides and free thiols using LC/MS. The loss of native disulfide and disulfide exchange was compared with model peptides derived from hGH.
Results
Loss of native disulfide in cyclic (cT20-T21) and linear peptides (T20-T21pep) derived from the C-terminal hGH disulfide during the first 60 min of reaction was greater than loss of the C-terminal disulfide in hGH itself. Of the thiols tested, glutathione (GSH) was the most reactive, forming the highest percentage of mixed disulfides in intact hGH and in the model peptides. At longer reaction times (>240 min), native disulfides in both hGH and cT20-T21 were regenerated. The fastest rates of regeneration were observed for Cys and the di- or tripeptide containing an Arg residue adjacent to Cys, suggesting that they may be useful in refolding.
Conclusions
Thiol-disulfide exchange reactions in hGH and related model peptides were influenced by higher order structure, by the size of the thiol reactant and by an Arg residue adjacent to Cys in the thiol reactant. Reduction of disulfide bonds in hGH did not affect higher order structure as measured by CD and HDX-MS.





Similar content being viewed by others
Abbreviations
- ACN:
-
Acetonitrile
- Cys:
-
Cysteine
- DTT:
-
Dithiotreitol
- FA:
-
Formic acid
- GSH:
-
Glutathione (reduced form)
- hGH:
-
Human growth hormone
- LC/MS:
-
Liquid chromatography / mass spectrometry
- MWCO:
-
Molecular weight cut-off
- PB:
-
Phosphate buffer
- qTOF:
-
Quadrupole time-of-flight
- TIC:
-
Total ion chromatogram
- XIC:
-
Extracted ion chromatogram
References
Zhang L, Chou CP, Moo-Young M. Disulfide bond formation and its impact on the biological activity and stability of recombinant therapeutic proteins produced by Escherichia coli expression system. Biotechnol Adv. 2011;29(6):923–9.
Summa D, Spiga O, Bernini A, Venditti V, Priora R, Frosali S, et al. Protein-thiol substitution or protein dethiolation by thiol/disulfide exchange reactions: the albumin model. Proteins. 2007;69(2):369–78.
Snyder GH, Cennerazzo MJ, Karalis AJ, Field D. Electrostatic influence of local cysteine environments on disulfide exchange kinetics. Biochemistry. 1981;20(23):6509–19.
Kuwajima K, Ikeguchi M, Sugawara T, Hiraoka Y, Sugai S. Kinetics of disulfide bond reduction in alpha-lactalbumin by dithiothreitol and molecular basis of superreactivity of the Cys6-Cys120 disulfide bond. Biochemistry. 1990;29(36):8240–9.
Zhang RM, Snyder GH. Dependence of formation of small disulfide loops in two-cysteine peptides on the number and types of intervening amino acids. J Biol Chem. 1989;264(31):18472–9.
Zhang T, Zhang J, Hewitt D, Tran B, Gao X, Qiu ZJ, et al. Identification and characterization of buried unpaired cysteines in a recombinant monoclonal IgG1 antibody. Anal Chem. 2012;84(16):7112–23.
Huh JH, White AJ, Brych SR, Franey H, Matsumura M. The identification of free cysteine residues within antibodies and a potential role for free cysteine residues in covalent aggregation because of agitation stress. J Pharm Sci. 2013;102(6):1701–11.
Kerr J, Schlosser JL, Griffin DR, Wong DY, Kasko AM. Steric effects in peptide and protein exchange with activated disulfides. Biomacromolecules. 2013;14(8):2822–9.
Trivedi MV, Laurence JS, Siahaan TJ. The role of thiols and disulfides on protein stability. Curr Protein Pept Sci. 2009;10(6):614–25.
Costantino HR, Langer R, Klibanov AM. Moisture-induced aggregation of lyophilized insulin. Pharm Res. 1994;11(1):21–9.
Correia IR. Stability of IgG isotypes in serum. MAbs. 2010;2(3):221–32.
Liu H, May K. Disulfide bond structures of IgG molecules: structural variations, chemical modifications and possible impacts to stability and biological function. MAbs. 2012;4(1):17–23.
Liu YD, Chen X, Enk JZ, Plant M, Dillon TM, Flynn GC. Human IgG2 antibody disulfide rearrangement in vivo. J Biol Chem. 2008;283(43):29266–72.
Costantino HR, Schwendeman SP, Langer R, Klibanov AM. Deterioration of lyophilized pharmaceutical proteins. Biochemistry (Mosc). 1998;63(3):357–63.
Browning JL, Mattaliano RJ, Chow EP, Liang SM, Allet B, Rosa J, et al. Disulfide scrambling of interleukin-2: HPLC resolution of the three possible isomers. Anal Biochem. 1986;155(1):123–8.
Wu SL, Leung D, Tretyakov L, Hu J, Guzzetta A, Wang YJ. The formation and mechanism of multimerization in a freeze-dried peptide. Int J Pharm. 2000;200(1):1–16.
Chandrasekhar S, Epling DE, Sophocleous AM, Topp EM. Thiol-disulfide exchange in peptides derived from human growth hormone. J Pharm Sci. 2014;103(4):1032–42.
Chandrasekhar S, Topp EM. Thiol-disulfide exchange in peptides derived from human growth hormone during lyophilization and storage in the solid state. J Pharm Sci. 2015;104(4):1291–302.
Wiesner J, Resemann A, Evans C, Suckau D, Jabs W. Advanced mass spectrometry workflows for analyzing disulfide bonds in biologics. Expert Rev Proteomics. 2015;12(2):115–23.
Wu SL, Jiang H, Lu Q, Dai S, Hancock WS, Karger BL. Mass spectrometric determination of disulfide linkages in recombinant therapeutic proteins using online LC-MS with electron-transfer dissociation. Anal Chem. 2009;81(1):112–22.
Liu F, van Breukelen B, Heck AJ. Facilitating protein disulfide mapping by a combination of pepsin digestion, electron transfer higher energy dissociation (EThcD), and a dedicated search algorithm SlinkS. Molec Cell Proteomics : MCP. 2014;13(10):2776–86.
Patra AK, Mukhopadhyay R, Mukhija R, Krishnan A, Garg LC, Panda AK. Optimization of inclusion body solubilization and renaturation of recombinant human growth hormone from Escherichia coli. Protein Expr Purif. 2000;18(2):182–92.
Zavialov AV, Gaestel M, Korpela T, Zav’yalov VP. Thiol/disulfide exchange between small heat shock protein 25 and glutathione. Biochim Biophys Acta. 1998;1388(1):123–32.
Junnila RK, Wu Z, Strasburger CJ. The role of human growth hormone’s C-terminal disulfide bridge. Growth Horm IGF Res. 2013;23(3):62–7.
Rabenstein DL, Weaver KH. Kinetics and Equilibria of the Thiol/Disulfide exchange reactions of Somatostatin with Glutathione. J Org Chem. 1996;61(21):7391–7.
Youngman KM, Spencer DB, Brems DN, DeFelippis MR. Kinetic analysis of the folding of human growth hormone. influence of disulfide bonds. J Biol Chem. 1995;270(34):19816–22.
Raman B, Ramakrishna T, Rao CM. Refolding of denatured and denatured/reduced lysozyme at high concentrations. J Biol Chem. 1996;271(29):17067–72.
Hansen RE, Ostergaard H, Winther JR. Increasing the reactivity of an artificial dithiol-disulfide pair through modification of the electrostatic milieu. Biochemistry. 2005;44(15):5899–906.
Wang YJ, Pearlman R. Stability and characterization of protein and peptide drugs : case histories. New York: Plenum Press; 1993. xxi, 353 p. p.
Okumura M, Saiki M, Yamaguchi H, Hidaka Y. Acceleration of disulfide-coupled protein folding using glutathione derivatives. FEBS J. 2011;278(7):1137–44.
Ferre F, Clote P. Disulfide connectivity prediction using secondary structure information and diresidue frequencies. Bioinformatics. 2005;21(10):2336–46.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors gratefully acknowledge financial support from NIH R01 GM085293. We also thank Dr. Jennifer Laurence (University of Kansas) for providing us with plasmid coding for hGH.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 2807 kb)
Rights and permissions
About this article
Cite this article
Chandrasekhar, S., Moorthy, B.S., Xie, R. et al. Thiol-Disulfide Exchange in Human Growth Hormone. Pharm Res 33, 1370–1382 (2016). https://doi.org/10.1007/s11095-016-1879-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-1879-3