Abstract
Purpose
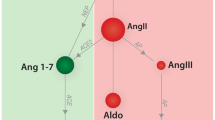
The objective of this research was to provide a comprehensive description of the effect of benazepril on the dynamics of the renin-angiotensin aldosterone system (RAAS) in dogs.
Methods
Blood specimens for renin activity (RA), angiotensin II (AII), and aldosterone (ALD) quantitation in plasma were drawn from 12 healthy adult beagle dogs randomly allocated to 2 treatment groups: (i) benazepril 5 mg PO, q24 h (n: 6) and (ii) placebo (n: 6), in a cross-over design. A mechanism-based pharmacokinetic/pharmacodynamic model, which includes the periodic nature of RA, AII, and ALD during placebo treatment and the subsequent changes in dynamics following repeated dosing with benazepril, was developed.
Results
The disposition kinetics of benazepril active metabolite, benazeprilat, was characterized using a saturable binding model to the angiotensin converting enzyme. The modulatory effect of benazeprilat on the RAAS was described using a combination of immediate response models. Our data show that benazepril noticeably influences the dynamics of the renin cascade, resulting in a substantial decrease in AII and ALD, while increasing RA throughout the observation span.
Conclusions
The model provides a quantitative framework for better understanding the effect of ACE inhibition on the dynamics of the systemic RAAS in dogs.







Similar content being viewed by others
Abbreviations
- ACE:
-
Angiotensin-converting enzyme
- AII:
-
Angiotensin II
- ALD:
-
Aldosterone
- ARA:
-
Aldosterone receptor antagonist
- ARB:
-
Angiotensin II receptor blocker
- BSV:
-
Between-subject variability (standard deviation of the random effect)
- CHF:
-
Congestive heart failure
- CV%:
-
Coefficient of variation (%)
- CVHD:
-
Chronic valvular heart disease
- EIA:
-
Enzyme immunoassay
- MB:
-
Mechanism-based
- OFV:
-
Objective function value
- PD:
-
Pharmacodynamic
- PK:
-
Pharmacokinetics
- PO:
-
Per os
- RA:
-
Renin activity
- RAAS:
-
Renin-angiotensin-aldosterone system
- RSE:
-
Relative standard error (equivalent to CV%)
- SD:
-
Standard deviation
- TMDD:
-
Target-mediated drug disposition
- WRES:
-
Weighted residuals
- C ij :
-
ng/ml, or nmol/l (nM) Predicted total benazeprilat concentration at time t ij for an individual i
- Δ ij :
-
pg/ml Predicted difference between AII concentrations during placebo treatment and those at corresponding times t ij after benazepril administration for an individual i
- T inf :
-
h Duration of the hypothetical infusion of benazepril into the depot compartment
- ka :
-
h−1 First-order rate constant representing the absorption of benazepril into the central compartment and its in vivo conversion to benazeprilat
- k 10 :
-
h−1 First-order rate constant of benazeprilat elimination from the central compartment
- k 1 :
-
nM−1.h−1 Second-order rate constant of association of the benazeprilat-ACE complex
- k 2 :
-
h−1 First-order rate constant of dissociation of the benazeprilat-ACE complex
- BS :
-
nM Maximal binding capacity to circulating ACE
- V c /F :
-
l/kg Apparent volume of distribution of benazeprilat
- Cl/F :
-
l.h−1/kg Apparent systemic clearance of benazeprilat
- E :
-
− Global extraction coefficient of benazeprilat
- M :
-
pg/ml or pg/ml.h−1 Mesor (daily average of rhythm)
- A :
-
pg/ml or pg/ml.h−1 Amplitude of the cosine function
- ψ :
-
h Acrophase (or time of peak) of the cosine function
- τ :
-
h Period of the cosine function
- I max (AII) :
-
− Maximum inhibition of AII production
- IC 50 (AII) :
-
ng/ml Benazeprilat concentration that produces half of the maximum inhibition of AII
- γ (AII) :
-
− Hill coefficient of the AII vs. benazeprilat effect curve
- E max (RA) :
-
− Maximum stimulatory effect on RA
- EC 50 (RA) :
-
pg/ml Difference in AII between placebo and benazepril-treated dogs for achieving 50% of the maximal stimulation of RA
- γ (RA) :
-
− Hill coefficient of the RA vs. AII effect curve
- I max (AL D) :
-
− Maximum inhibition of ALD production
- IC 50 (ALD) :
-
pg/ml Difference in AII between placebo and benazepril-treated dogs for achieving 50% of the maximal inhibition of ALD
- γ (ALD) :
-
− Hill coefficient of the ALD vs. AII effect curve
REFERENCES
Atkins C, Bonagura J, Ettinger S, Fox P, Gordon S, Haggstrom J, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med. 2009;23(6):1142–50.
Guglielmini C. Cardiovascular diseases in the ageing dog: diagnostic and therapeutic problems. Vet Res Commun. 2003;27(1):555–60.
Sayer MB, Atkins CE, Fujii Y, Adams AK, DeFrancesco TC, Keene BW. Acute effect of pimobendan and furosemide on the circulating renin-angiotensin-aldosterone system in healthy dogs. J Vet Intern Med. 2009;23(5):1003–6.
Roig E, Perez-Villa F, Morales M, Jiménez W, Orús J, Heras M, et al. Clinical implications of increased plasma angiotensin II despite ACE inhibitor therapy in patients with congestive heart failure. Eur Heart J. 2000;21(1):53–7.
Güder G, Bauersachs J, Frantz S, Weismann D, Allolio B, Ertl G, et al. Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation. 2007;115(13):1754–61.
Tan LB, Jalil JE, Pick R, Janicki JS, Weber KT. Cardiac myocyte necrosis induced by angiotensin II. Circ Res. 1991;69(5):1185–95.
Shimizu M, Tanaka R, Fukuyama T, Aoki R, Orito K, Yamane Y. Cardiac remodeling and angiotensin II-forming enzyme activity of the left ventricle in hamsters with chronic pressure overload induced by ascending aortic stenosis. J Vet Med Sci. 2006;68(3):271–6.
BENCH (BENazepril in Canine Heart disease) Study Group. The effect of benazepril on survival times and clinical signs of dogs with congestive heart failure: Results of a multicenter, prospective, randomized, double-blinded, placebo-controlled, long-term clinical trial. J Vet Cardiol. 1999;1(1):7–18.
Webb R. Benazepril. Cardiovasc Drug Rev. 1990;8(2):89–104.
Toutain PL, Lefebvre HP. Pharmacokinetics and pharmacokinetic/pharmacodynamic relationships for angiotensin-converting enzyme inhibitors. J Vet Pharmacol Ther. 2004;27(6):515–25.
Mochel JP, Peyrou M, Fink M, Strehlau G, Mohamed R, Giraudel JM, et al. Capturing the dynamics of systemic Renin-Angiotensin-Aldosterone System (RAAS) peptides heightens the understanding of the effect of benazepril in dogs. J Vet Pharmacol Ther. 2013;36(2):174–80.
Lijnen P, Staessen J, Fagard R, Amery A. Increase in plasma aldosterone during prolonged captopril treatment. Am J Cardiol. 1982;49(6):1561–3.
Van de Wal RM, Plokker HW, Lok DJ, Boomsma F, van der Horst FA, van Veldhuisen DJ, et al. Determinants of increased angiotensin II levels in severe chronic heart failure patients despite ACE inhibition. Int J Cardiol. 2006;106(3):367–72.
Sisson DD. Neuroendocrine evaluation of cardiac disease. Vet Clin North Am Small Anim Pract. 2004;34(5):1105–26.
Muller AF, Manning EL, Riondel AM. Influence of position and activity on the secretion of aldosterone. Lancet. 1958;1(7023):711–3.
Mochel JP, Fink M, Peyrou M, Desevaux C, Deurinck M, Giraudel JM, et al. Chronobiology of the renin-angiotensin-aldosterone system in dogs: relation to blood pressure and renal physiology. Chronobiol Int. 2013;30(9):1144–59.
Mochel JP, Fink M, Bon C, Peyrou M, Bieth B, Desevaux C, et al. Influence of feeding schedules on the chronobiology of renin activity, urinary electrolytes and blood pressure in dogs. Chronobiol Int. 2014;31(5):715–30.
Sheiner LB, Ludden TM. Population pharmacokinetics/dynamics. Annu Rev Pharmacol Toxicol. 1992;32:185–209.
Lindbom L, Ribbing J, Jonsson EN. Perl-speaks-NONMEM (PsN)—A Perl module for NONMEM related programming. Comput Methods Programs Biomed. 2004;75:85–94.
Jonsson EN, Karlsson MO. Xpose—An S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1999;58:51–64.
Lees KR, Kelman AW, Reid JL, Whiting B. Pharmacokinetics of an ACE inhibitor, S-9780, in man: evidence of tissue binding. J Pharmacokinet Biopharm. 1989;17(5):529–50.
Toutain PL, Lefebvre HP, King JN. Benazeprilat disposition and effect in dogs revisited with a pharmacokinetic/pharmacodynamic modeling approach. J Pharmacol Exp Ther. 2000;292(3):1087–93.
Picard-Hagen N, Gayrard V, Alvinerie M, Smeyers H, Ricou R, Bousquet-Melou A, et al. A nonlabeled method to evaluate cortisol production rate by modeling plasma CBG-free cortisol disposition. Am J Physiol Endocrinol Metab. 2001;281(5):E946–56.
Oppong SY, Hooper NM. Characterization of a secretase activity which releases angiotensin-converting enzyme from the membrane. Biochem J. 1993;292(Pt 2):597–603.
Levy G. Pharmacologic target-mediated drug disposition. Clin Pharmacol Ther. 1994;56(3):248–52.
Toutain PL, Bousquet-Melou A. Plasma terminal half-life. J Vet Pharmacol Ther. 2004;27(6):427–39.
Nicholls MG, Richards AM, Crozier IG, Espiner EA, Ikram H. Cardiac natriuretic peptides in heart failure. Ann Med. 1993;25(6):503–5.
Knowlen GG, Kittleson MD, Nachreiner RF, Eyster GE. Comparison of plasma aldosterone concentration among clinical status groups of dogs with chronic heart failure. J Am Vet Med Assoc. 1983;183(9):991–6.
Häggström J, Hansson K, Karlberg BE, Kvart C, Madej A, Olsson K. Effects of long-term treatment with enalapril or hydralazine on the renin-angiotensin-aldosterone system and fluid balance in dogs with naturally acquired mitral valve regurgitation. Am J Vet Res. 1996;57(11):1645–52.
Koch J, Pedersen HD, Jensen AL, Flagstad A, Poulsen K, Bie P. Short term effects of acute inhibition of the angiotensin-converting enzyme on the renin-angiotensin system and plasma atrial natriuretic peptide in healthy dogs fed a low-sodium diet versus a normal-sodium diet. Zentralbl Veterinarmed A. 1994;41(2):121–7.
Geary KM, Hunt MK, Peach MJ, Gomez RA, Carey RM. Effects of angiotensin converting enzyme inhibition, sodium depletion, calcium, isoproterenol, and angiotensin II on renin secretion by individual renocortical cells. Endocrinology. 1992;131(4):1588–94.
Azizi M, Bissery A, Peyrard S, Guyene TT, Ozoux ML, Floch A, et al. Pharmacokinetics and pharmacodynamics of the vasopeptidase inhibitor AVE7688 in humans. Clin Pharmacol Ther. 2006;17(1):13–8.
Hong Y, Dingemanse J, Mager DE. Pharmacokinetic/pharmacodynamic modeling of renin biomarkers in subjects treated with the renin inhibitor aliskiren. Clin Pharmacol. 2008;84(1):136–43.
Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown Jr EJ, Cuddy TE, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327(10):669–77.
King JN, Mauron C, Kaiser G. Pharmacokinetics of the active metabolite of benazepril, benazeprilat, and inhibition of plasma angiotensin-converting enzyme activity after single and repeated administrations to dogs. Am J Vet Res. 1995;56(12):1620–8.
Jorde UP, Vittorio T, Katz SD, Colombo PC, Latif F, Le Jemtel TH. Elevated plasma aldosterone levels despite complete inhibition of the vascular angiotensin-converting enzyme in chronic heart failure. Circulation. 2002;106(9):1055–7.
Fyhrquist F, Saijonmaa O. Renin-angiotensin system revisited. J Intern Med. 2008;264(3):224–36.
Cleland JG, Dargie HJ, Robertson JI. Angiotensin converting enzyme inhibition in heart failure. Br J Clin Pharmacol. 1984;18 Suppl 2:157S–60.
McCaa RE, Guyton AC, Young DB, McCaa CS. Role of angiotensin II in the regulation of aldosterone biosynthesis. Adv Exp Med Biol. 1980;130:227–55.
Swedberg K, Eneroth P, Kjekshus J, Wilhelmsen L. Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. CONSENSUS Trial Study Group. Circulation. 1990;82(5):1730–6.
MacFadyen RJ, Lee AF, Morton JJ, Pringle SD, Struthers AD. How often are angiotensin II and aldosterone concentrations raised during chronic ACE inhibitor treatment in cardiac failure? Heart. 1999;82(1):57–61.
Rocha R, Chander PN, Zuckerman A, Stier CT Jr. Role of aldosterone in renal vascular injury in stroke-prone hypertensive rats. Hypertension. 1999;33(1 Pt 2):232–7.
Latini R, Masson S, Anand I, Salio M, Hester A, Judd D, et al. The comparative prognostic value of plasma neurohormones at baseline in patients with heart failure enrolled in Val-HeFT. Eur Heart J. 2004;25(4):292–9.
Bernay F, Bland JM, Häggström J, Baduel L, Combes B, Lopez A, Kaltsatos V. Efficacy of spironolactone on survival in dogs with naturally occurring mitral regurgitation caused by myxomatous mitral valve disease. J Vet Intern Med. 2010;24(2):331–41.
Ovaert P, Elliott J, Bernay F, Guillot E, Bardon T. Aldosterone receptor antagonists: how cardiovascular actions may explain their beneficial effects in heart failure. J Vet Pharmacol Ther. 2010;33(2):109–17.
Naruse M, Tanabe A, Sato A, Takagi S, Tsuchiya K, Imaki T, et al. Aldosterone breakthrough during angiotensin II receptor antagonist therapy in stroke-prone spontaneously hypertensive rats. Hypertension. 2002;40(1):28–33.
Rousseau MF, Gurné O, Duprez D, Van Mieghem W, Robert A, Ahn S, et al. Beneficial neurohormonal profile of spironolactone in severe congestive heart failure: results from the RALES neurohormonal substudy. J Am Coll Cardiol. 2002;40(9):1596–601.
The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316(23):1429–35.
Brown NJ, Vaughan DE. Angiotensin-converting enzyme inhibitors. Circulation. 1998;97(14):1411–20.
Azibani F, Fazal L, Chatziantoniou C, Samuel JL, Delcayre C. Hypertension-induced fibrosis: a balance story. Ann Cardiol Angeiol. 2012;61(3):150–5.
Mishina M, Watanabe T. Development of hypertension and effects of benazepril hydrochloride in a canine remnant kidney model of chronic renal failure. J Vet Med Sci. 2008;70(5):455–60.
Kjolby MJ, Kompanowska-Jezierska E, Wamberg S, Bie P. Effects of sodium intake on plasma potassium and renin angiotensin aldosterone system in conscious dogs. Acta Physiol Scand. 2005;184(3):225–34.
Mochel JP, Fink M. Response to letter from Atkins et al. J Vet Pharmacol Ther. 2012;35(5):516–8.
ACKNOWLEDGMENTS AND DISCLOSURES
These investigations were conducted at the Centre de Recherche Sante Animale SA (CRA) of Novartis Animal Health, located in St-Aubin, Switzerland.
With the exception of Prof. Meindert Danhof, the authors of the manuscript are Novartis employees. The experiments were supported by Novartis Animal Health, Basel, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 15 kb)
ESM 2
(DOCX 19 kb)
ESM 3
Mirror plots of the full pharmacokinetic/pharmacodynamic model. (A) Benazeprilat, (B) Angiotensin II, (C) Renin activity, (D) Aldosterone. Comparison of predictions (individual, population) obtained from the raw observations and the simulated datasets using Xpose version 4.1. LNDV.1: observations (log scale) from the original or the simulated datafile. The mirror plots option of Perl-speaks-NONMEM was used to produce 3 simulation table files. Using the same model structure, these simulated datasets were then used as input files to derive a new set of parameter estimates. The dispersion pattern obtained from the 3 simulated datasets ‘mirrored’ the diagnostic plots obtained with the original data, which indicates that the model structure is well-characterized. (GIF 139 kb)
(GIF 139 kb)
(GIF 152 kb)
(GIF 136 kb)
Rights and permissions
About this article
Cite this article
Mochel, J.P., Fink, M., Peyrou, M. et al. Pharmacokinetic/Pharmacodynamic Modeling of Renin-Angiotensin Aldosterone Biomarkers Following Angiotensin-Converting Enzyme (ACE) Inhibition Therapy with Benazepril in Dogs. Pharm Res 32, 1931–1946 (2015). https://doi.org/10.1007/s11095-014-1587-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1587-9