Abstract
Purpose
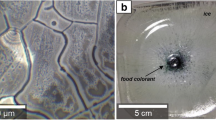
The potential contribution of protein aggregates to the unwanted immunogenicity of protein pharmaceuticals is a major concern. In the present study a murine monoclonal antibody was utilized to study the immunogenicity of different types of aggregates in mice. Samples containing defined types of aggregates were prepared by processes such as stirring, agitation, exposure to ultraviolet (UV) light and exposure to elevated temperatures.
Methods
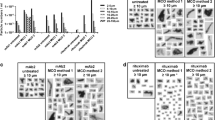
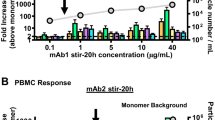
Aggregates were analyzed by size-exclusion chromatography, light obscuration, turbidimetry, infrared (IR) spectroscopy and UV spectroscopy. Samples were separated into fractions based on aggregate size by asymmetrical flow field-flow fractionation or by centrifugation. Samples containing different types and sizes of aggregates were subsequently administered to C57BL/6 J and BALB/c mice, and serum was analyzed for the presence of anti-IgG1, anti-IgG2a, anti-IgG2b and anti-IgG3 antibodies. In addition, the pharmacokinetic profile of the murine antibody was investigated.
Results
In this study, samples containing high numbers of different types of aggregates were administered in order to challenge the in vivo system. The magnitude of immune response depends on the nature of the aggregates. The most immunogenic aggregates were of relatively large and insoluble nature, with perturbed, non-native structures.
Conclusion
This study shows that not all protein drug aggregates are equally immunogenic.





Similar content being viewed by others
Abbreviations
- ADAs:
-
Anti-drug antibodies
- AF4:
-
Asymmetric flow field flow fractionation
- ATR:
-
Attenuated total reflection
- AUC:
-
Area under the curve
- DMSO:
-
Dimethylsulfoxide
- ELISA:
-
Enzyme-linked immunosorbent assay
- FNU:
-
Formazine nephelometric units
- HRP:
-
Horseradish peroxidase
- IR:
-
Infrared spectroscopy
- mAb1:
-
Monoclonal antibody
- MALLS:
-
Multi angle laser light scattering
- PBS:
-
Phosphate buffered saline
- RI:
-
Refractive index
- UV:
-
Ultraviolet light
References
Berger M, Shankar V, Vafai A. Therapeutic applications of monoclonal antibodies. Am J Med Sci. 2002;324(1):14–30.
Koren E, Smith HW, Shores E, Shankar G, Finco-Kent D, Rup B, et al. Recommendations on risk-based strategies for detection and characterization of antibodies against biotechnology products. J Immunol Methods. 2008;333(1–2):1–9. Epub 2008/02/16.
Tamilvanan S, Raja NL, Sa B, Basu SK. Clinical concerns of immunogenicity produced at cellular levels by biopharmaceuticals following their parenteral administration into human body. J Drug Target. 2010;18(7):489–98.
Schellekens H. Immunogenicity of therapeutic proteins. Nephrol Dial Transplant. 2003;18(7):1257–9.
Singh SK. Impact of product-related factors on immunogenicity of biotherapeutics. J Pharm Sci. 2011;100(2):354–87.
Yanai H, Hanauer SB. Assessing response and loss of response to biological therapies in IBD. Am J Gastroenterol. 2011;106(4):685–98.
Swanson SJ, editor. Immunogenicity of Therapeutic Proteins. Hoboken: Wiley; 2010.
Shankar G, Pendley C, Stein KE. A risk-based bioanalytical strategy for the assessment of antibody immune responses against biological drugs. Nat Biotechnol. 2007;25(5):555–61. Epub 2007/05/08.
Schellekens H. Immunogenicity of protein therapeutics, or how to make antibodies without T-cells. Inflamm Res. 2007;56:S351–S2.
Schellekens H. Factors influencing the immunogenicity of therapeutic proteins. Nephrol Dial Transplant. 2005;20:3–9.
Schellekens H, Casadevall N. Immunogenicity of recombinant human proteins: causes and consequences. J Neurol. 2004;251:4–9.
Schellekens H. The immunogenicity of biopharmaceuticals. Neurology. 2003;61(9):S11–S2.
Schellekens H. Relationship between biopharmaceutical immunogenicity of epoetin alfa and pure red cell aplasia. Curr Med Res Opin. 2003;19(5):433–4.
Schellekens H. Immunogenicity of therapeutic proteins: clinical implications and future prospects. Clin Ther. 2002;24(11):1720–40.
Rosenberg AS, Worobec A. A risk-based approach to immunogenicity concerns of therapeutic protein products part 1 considering consequences of the immune response to a protein. Biopharm Int. 2004;17(11):22−+.
Rosenberg A, editor. FDA Perspective on Immunogenicity Testing- A Risk Based Analysis. Bethesda, MD; 2003.
Petersen B, Bendtzen K, Koch-Henriksen N, Ravnborg M, Ross C, Sorensen PS. Persistence of neutralizing antibodies after discontinuation of IFN beta therapy in patients with relapsing-remitting multiple sclerosis. Mult Scler. 2006;12(3):247–52.
De Groot AS, Scott DW. Immunogenicity of protein therapeutics. Trends Immunol. 2007;28(11):482–90.
Antonelli G, Dianzani F. Development of antibodies to interferon beta in patients: technical and biological aspects. Eur Cytokine Netw. 1999;10(3):413–22.
Schellekens H. Immunologic mechanisms of EPO-associated pure red cell aplasia. Best Pract Res Clin Haematol. 2005;18(3):473–80.
Schernthaner G. Immunogenicity and allergenic potential of animal and human insulins. Diabetes Care. 1993;16 Suppl 3:155–65.
Goodin DS, Frohman EM, Hurwitz B, O’Connor PW, Oger JJ, Reder AT, et al. Neutralizing antibodies to interferon beta: assessment of their clinical and radiographic impact: an evidence report: report of the therapeutics and technology assessment subcommittee of the American academy of neurology. Neurology. 2007;68(13):977–84.
Ring J, Stephan W, Brendel W. Anaphylactoid reactions to infusions of plasma-protein and human-serum albumin - role of aggregated proteins and of stabilizers added during production. Clin Allergy. 1979;9(1):89–97.
Christian CL. Studies of aggregated γ-globulin: II effect in vivo. J Immunol. 1960;84(1):117–21.
Roskos LK, Davis CG, Schwab GM. The clinical pharmacology of therapeutic monoclonal antibodies. Drug Dev Res. 2004;61(3):108–20.
Pendley C, Schantz A, Wagner C. Immunogenicity of therapeutic monoclonal antibodies. Curr Opin Mol Ther. 2003;5(2):172–9.
Hermeling S, Aranha L, Damen JMA, Slijper M, Schellekens H, Crommelin DJA, et al. Structural characterization and immunogenicity in wild-type and immune tolerant mice of degraded recombinant human interferon Alpha2b. Pharm Res. 2005;22(12):1997–2006.
Braun A, Kwee L, Labow MA, Alsenz J. Protein aggregates seem to play a key role among the parameters influencing the antigenicity of interferon alpha (IFN-alpha ) in normal and transgenic mice. Pharm Res. 1997;14(10):1472–8.
Van Beers MMC, Gilli F, Schellekens H, Randolph TW, Jiskoot W. Immunogenicity of recombinant human interferon beta interacting with particles of glass, metal, and polystyrene. J Pharm Sci. 2012;101(1):187–99.
Hesterberg LK, Seefeldt MB, Carpenter JF, Randolph TW. High-Hydrostatic pressure refolding of proteins. Genet Eng News. 2005;25(4):46–7.
Fradkin AH, Carpenter JF, Randolph TW. Immunogenicity of aggregates of recombinant human growth hormone in mouse models. J Pharm Sci. 2009;98(9):3247–64.
van Beers MMC, Sauerborn M, Gilli F, Brinks V, Schellekens H, Jiskoot W. Aggregated recombinant human interferon beta induces antibodies but no memory in immune-tolerant transgenic mice. Pharm Res. 2010;27(9):1812–24.
Fradkin AH, Carpenter JF, Randolph TW. Glass particles as an adjuvant: a model for adverse immunogenicity of therapeutic proteins. J Pharm Sci. 2011;100(11):4953–64.
Brinks V, Jiskoot W, Schellekens H. Immunogenicity of therapeutic proteins: the use of animal models. Pharm Res. 2011;28(10):2379–85.
Hwang WYK, Foote J. Immunogenicity of engineered antibodies. Methods (San Diego, CA, U S). 2005;36(1):3–10.
Schoeneich C. Light-induced oxidation and aggregation of proteins: potential immunogenicity consequences. Workshop on Protein Aggregation and Immunogenicity; July, 2010; Breckenridge, CO, July 20–22, 2010
Wang W. Protein aggregation and its inhibition in biopharmaceutics. Int J Pharm. 2005;289(1–2):1–30.
Watanabe H, Numata K, Ito T, Takagi K, Matsukawa A. Innate immune response in Th1- and Th2-dominant mouse strains. Shock. 2004;22(5):460–6.
PhEur 2.2.1. Clarity and degree of opalescence of liquids. European Directorate for the Quality of Medicine (EDQM). 7th edition; 2011.
PhEur 0169. Monograph “Water for injections”. European Directorate for the Quality of Medicine (EDQM). 7th edition; 2011.
Dintzis HM, Dintzis RZ, Vogelstein B. Molecular determinants of immunogenicity: the immunon model of immune response. Proc Natl Acad Sci U S A. 1976;73(10):3671–5.
Martin RM, Brady JL, Lew AM. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J Immunol Methods. 1998;212(2):187–92.
Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004;82(5):488–96.
Freitag AJ, Wittmann K, Winter G, Myschik J. The preparative use of flow field-flow fractionation. LCGC Europe. 2011;24(3):134.
Shomali M, Freitag A, Engert J, Siedler M, Kaymakcalan Z, Winter G, et al. Antibody responses in mice to particles formed from adsorption of a murine monoclonal antibody onto glass microparticles. J Pharm Sci. 2014;103(1):78–89.
Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164(12):6166–73.
Coutelier JP, Van der Logt JTM, Heessen FWA, Vink A, Van Snick A. Virally induced modulation of murine IgG antibody subclasses. J Exp Med. 1988;168(6):2373–8.
Stevens TL, Bossie A, Sanders VM, Fernandez-Botran R, Coffman RL, Mosmann TR, et al. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature (London). 1988;334(6179):255–8.
Ramakrishna C, Ravi V, Desai A, Subbakrishna DK, Shankar SK, Chandramuki A. T helper responses to japanese encephalitis virus infection are dependent on the route of inoculation and the strain of mouse used. J Gen Virol. 2003;84(6):1559–67.
Feltquate DM, Heaney S, Webster RG, Robinson HL. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J Immunol. 1997;158(5):2278–84.
Hermeling S, Schellekens H, Maas C, Gebbink MFBG, Crommelin DJA, Jiskoot W. Antibody response to aggregated human interferon alpha2b in wild-type and transgenic immune tolerant mice depends on type and level of aggregation. J Pharm Sci. 2006;95(5):1084–96.
Vollmar D. Immunologie - Grundlagen und Wirkstoffe. 1st ed. München, Frankfurt am Main: Wissenschaftliche Verlagsgesellschaft mbH Stuttgart; 2005.
Haley PJ. Species differences in the structure and function of the immune system. Toxicology. 2003;188(1):49–71.
Filipe V, Que I, Carpenter J, Löwik C, Jiskoot W. In vivo fluorescence imaging of IgG1 aggregates after subcutaneous and intravenous injection in mice. Pharm Res. 2014;31(1):216–27.
Kijanka G, Prokopowicz M, Schellekens H, Brinks V. Influence of aggregation and route of injection on the biodistribution of mouse serum albumin. PLoS One. 2014;9(1):1–9.
Acknowledgments and Disclosures
The authors would like to thank AbbVie Inc. for providing the protein and financial support.
Disclosure of Potential Conflicts of Interest
Zehra Kaymakcalan and Michael Siedler are employees of AbbVie and are Abbvie stockholders.
The University of Colorado and the Ludwig-Maximilians-University Munich received research funds from AbbVie Inc. (former Abbott Laboratories) to conduct the study.
AbbVie (former Abbott Laboratories) provided financial support, provided the murine antibody used in this study, as well as resources to support the in-vivo studies and the bioanalytical characterization.
Furthermore, AbbVie authors were involved in study design, research, analysis, data collection, interpretation of data, reviewing and approving the publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Freitag, A.J., Shomali, M., Michalakis, S. et al. Investigation of the Immunogenicity of Different Types of Aggregates of a Murine Monoclonal Antibody in Mice. Pharm Res 32, 430–444 (2015). https://doi.org/10.1007/s11095-014-1472-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1472-6