Abstract
Purpose
Neutropenia is a severe adverse-event of chemotherapeutics. Neutrophils (ANC) are mainly regulated by granulocyte colony stimulating factor (G-CSF). The aim was to characterize the dynamics between endogenous G-CSF and ANC over time following chemotherapy.
Methods
Endogenous G-CSF and ANC were monitored in forty-nine breast cancer patients treated with sequential adjuvant 5-fluorouracil–epirubicin–cyclophosphamide and docetaxel.
Results
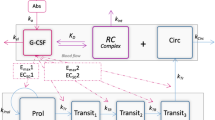
During treatment courses ANC was transiently decreased and was reflected in an endogenous G-CSF increase, which was well described by a semi-mechanistic model including control mechanisms; when G-CSF concentrations increased the proliferation rate increased and the bone maturation time reduced for ANC. Subsequently, ANC in the circulation increased leading to increased elimination of G-CSF. Additionally, a non-specific elimination for G-CSF was quantified. The ANC-dependent elimination contributed to 97% at baseline and 49% at an ANC of 0.1 · 109/L to the total G-CSF elimination.
Conclusion
The integrated G-CSF–myelosuppression model captured the initial rise in endogenous G-CSF following chemotherapy-induced neutropenia and the return to baseline of G-CSF and ANC. The model supported the self-regulatory properties of the system and may be a useful tool for further characterization of the biological system and in optimization of chemotherapy treatment.





Similar content being viewed by others
Abbreviations
- AAG:
-
Alpha-1 acid glycoprotein
- ALB:
-
Albumin
- ALT:
-
Alanine aminotransferase
- ANC:
-
Absolute neutrophil count
- ANC0 :
-
Baseline neutrophil count
- AP:
-
Alkaline phosphate
- AST:
-
Aspartate aminotransferase
- BSA:
-
Body surface area
- BSV:
-
Between subject variability
- CLCR :
-
Creatinine clearance
- CRP:
-
C-reactive protein
- CV%:
-
Coefficient of variation
- DOSEcort :
-
Amount of cortisol-induced G-CSF release
- FEC:
-
5-flourouracil-epirubicin-cyclophosphamide
- FOCE:
-
First-order conditional estimation method
- G-CSF:
-
Granulocyte colony stimulating factor
- GCSF0 :
-
Baseline G-CSF
- IL-6:
-
Interleukin 6
- kANC :
-
ANC-dependent elimination rate constant
- ke :
-
Non-specific elimination rate constant
- MMT:
-
Mean bone marrow maturation time of neutrophils
- MMTFEC :
-
Mean bone marrow maturation time of neutrophils following FEC treatment
- OFV:
-
Objective function value
- PK:
-
Pharmacokinetics
- PKPD:
-
Pharmacokinetic-pharmacodynamic
- rh-GCSF:
-
Recombinant G-CSF
- RSE:
-
Relative standard error
- SLOPE5-FU :
-
Linear drug effect parameters for 5-FU
- SLOPEcyclo :
-
Linear drug effect parameters for 4-hydroxy cyclophosphamide
- SLOPEdoce :
-
Linear drug effect parameters for docetaxel
- SLOPEepi, :
-
Linear drug effect parameters for epirubicin
- t1/2 circ:
-
Half-life of neutrophils in circulation
- t1/2 cort:
-
Half-life of cortisol-induced G-CSF release
- VPC:
-
Visual predictive check
- 4-OHCP:
-
4-hydroxy cyclophosphamide
- 5-FU:
-
5-flourouracil
- β:
-
Feedback of G-CSF on transit time
- γ:
-
Feedback of G-CSF on neutrophil proliferation
- θMMT-doce :
-
Change in MMT following docetaxel compared to FEC
References
Crawford J, Dale DC, Kuderer NM, Culakova E, Poniewierski MS, Wolff D, et al. Risk and timing of neutropenic events in adult cancer patients receiving chemotherapy: the results of a prospective nationwide study of oncology practice. J Natl Compr Cancer Netw JNCCN. 2008;6(2):109–18.
Lyman GH, Dale DC, Friedberg J, Crawford J, Fisher RI. Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin’s lymphoma: a nationwide study. J Clin Oncol Off J Am Soc Clin Oncol. 2004;22(21):4302–11.
Colleoni M, Price K, Castiglione-Gertsch M, Goldhirsch A, Coates A, Lindtner J, et al. Dose–response effect of adjuvant cyclophosphamide, methotrexate, 5-fluorouracil (CMF) in node-positive breast cancer. International Breast Cancer Study Group. Eur J Cancer Oxf Engl 1990. 1998 Oct;34(11):1693–700.
Nicola NA. Granulocyte colony-stimulating factor. Immunol Ser. 1990;49:77–109.
Roberts AW. G-CSF: a key regulator of neutrophil production, but that’s not all! Growth Factors Chur Switz. 2005;23(1):33–41.
Hareng L, Hartung T. Induction and regulation of endogenous granulocyte colony-stimulating factor formation. Biol Chem. 2002;383(10):1501–17.
Reisbach G, Kamp T, Welzl G, Geiz C, Abedinpour F, Lodri A, et al. Regulated plasma levels of colony-stimulating factors, interleukin-6 and interleukin-10 in patients with acute leukaemia and non-hodgkin’s lymphoma undergoing cytoreductive chemotherapy. Br J Haematol. 1996;92(4):907–12.
Kawakami M, Tsutsumi H, Kumakawa T, Abe H, Hirai M, Kurosawa S, et al. Levels of serum granulocyte colony-stimulating factor in patients with infections. Blood. 1990;76(10):1962–4.
Friberg LE, Karlsson MO. Mechanistic models for myelosuppression. Invest New Drugs. 2003;21(2):183–94.
Soto E, Staab A, Doege C, Freiwald M, Munzert G, Trocóniz IF. Comparison of different semi-mechanistic models for chemotherapy-related neutropenia: application to BI 2536 a Plk-1 inhibitor. Cancer Chemother Pharmacol. 2011;68(6):1517–27.
Friberg LE, Henningsson A, Maas H, Nguyen L, Karlsson MO. Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin Oncol Off J Am Soc Clin Oncol. 2002;20(24):4713–21.
Hayashi N, Kinoshita H, Yukawa E, Higuchi S. Pharmacokinetic and pharmacodynamic analysis of subcutaneous recombinant human granulocyte colony stimulating factor (lenograstim) administration. J Clin Pharmacol. 1999;39(6):583–92.
Krzyzanski W, Ramakrishnan R, Jusko WJ. Basic pharmacodynamic models for agents that alter production of natural cells. J Pharmacokinet Biopharm. 1999;27(5):467–89.
Sugiura M, Yamamoto K, Sawada Y, Iga T. Pharmacokinetic/pharmacodynamic analysis of neutrophil proliferation induced by recombinant granulocyte colony-stimulating factor (rhG-CSF): comparison between intravenous and subcutaneous administration. Biol Pharm Bull. 1997;20(6):684–9.
Wang B, Ludden TM, Cheung EN, Schwab GG, Roskos LK. Population pharmacokinetic-pharmacodynamic modeling of filgrastim (r-metHuG-CSF) in healthy volunteers. J Pharmacokinet Pharmacodyn. 2001;28(4):321–42.
Roskos LK, Lum P, Lockbaum P, Schwab G, Yang B-B. Pharmacokinetic/pharmacodynamic modeling of pegfilgrastim in healthy subjects. J Clin Pharmacol. 2006;46(7):747–57.
Krzyzanski W, Wiczling P, Lowe P, Pigeolet E, Fink M, Berghout A, et al. Population modeling of filgrastim PK-PD in healthy adults following intravenous and subcutaneous administrations. J Clin Pharmacol. 2010;50(9 Suppl):101S–12.
Sugiura M, Ohno Y, Yamada Y, Suzuki H, Iga T. Pharmacokinetic/pharmacodynamic analysis of neutrophil proliferation induced by rhG-CSF in patients receiving antineoplastic drugs. Yakugaku Zasshi. 2004;124(9):599–604.
Pastor ML, Laffont CM, Gladieff L, Schmitt A, Chatelut E, Concordet D. Model-Based Approach to Describe G-CSF Effects in Carboplatin-Treated Cancer Patients. Pharm Res. 2013 Jun 26
Yang B-B, Lum PK, Hayashi MM, Roskos LK. Polyethylene glycol modification of filgrastim results in decreased renal clearance of the protein in rats. J Pharm Sci. 2004;93(5):1367–73.
Roskos LK, Cheung EN, Vincent M, Foote M, Morstyn G. Pharmacology of filgrastim (r-metHuG-CSF). Filgrastim R-MetHuG-CSF Clin Pract N Y NY Marcel Dekker. 1998;41–9.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41.
Bruno R, Vivier N, Vergniol JC, De Phillips SL, Montay G, Sheiner LB. A population pharmacokinetic model for docetaxel (Taxotere): model building and validation. J Pharmacokinet Biopharm. 1996;24(2):153–72.
Sandström M, Lindman H, Nygren P, Johansson M, Bergh J, Karlsson MO. Population analysis of the pharmacokinetics and the haematological toxicity of the fluorouracil-epirubicin-cyclophosphamide regimen in breast cancer patients. Cancer Chemother Pharmacol. 2006;58(2):143–56.
Urien S, Rezaí K, Lokiec F. Pharmacokinetic modelling of 5-FU production from capecitabine–a population study in 40 adult patients with metastatic cancer. J Pharmacokinet Pharmacodyn. 2005;32(5–6):817–33.
Smith C, Beutler E, Lichtman M, Coller B, Kipps T. Williams hematology. Lichtman M Kipps T Seligsohn U Kaushansky K JT P Eds. 2010;
Hansson EK, Wallin JE, Lindman H, Sandström M, Karlsson MO, Friberg LE. Limited inter-occasion variability in relation to inter-individual variability in chemotherapy-induced myelosuppression. Cancer Chemother Pharmacol. 2010;65(5):839–48.
Gisleskog PO, Karlsson MO, Beal SL. Use of prior information to stabilize a population data analysis. J Pharmacokinet Pharmacodyn. 2002;29(5–6):473–505.
Quartino AL, Friberg LE, Karlsson MO. A simultaneous analysis of the time-course of leukocytes and neutrophils following docetaxel administration using a semi-mechanistic myelosuppression model. Invest New Drugs. 2012;30(2):833–45.
Ozawa K, Minami H, Sato H. Population pharmacokinetic and pharmacodynamic analysis for time courses of docetaxel-induced neutropenia in Japanese cancer patients. Cancer Sci. 2007;98(12):1985–92.
Soto E, Staab A, Freiwald M, Munzert G, Fritsch H, Döge C, et al. Prediction of neutropenia-related effects of a new combination therapy with the anticancer drugs BI 2536 (a Plk1 Inhibitor) and pemetrexed. Clin Pharmacol Ther. 2010;88(5):660–7.
Czerwinski AW, Czerwinski AB, Whitsett TL, Clark ML. Effects of a single, large, intravenous injection of dexamethasone. Clin Pharmacol Ther. 1972;13(5):638–42.
Mager DE, Lin SX, Blum RA, Lates CD, Jusko WJ. Dose equivalency evaluation of major corticosteroids: pharmacokinetics and cell trafficking and cortisol dynamics. J Clin Pharmacol. 2003;43(11):1216–27.
Friberg LE, Brindley CJ, Karlsson MO, Devlin AJ. Models of schedule dependent haematological toxicity of 2’-deoxy-2’-methylidenecytidine (DMDC). Eur J Clin Pharmacol. 2000;56(8):567–74.
Karlsson MO, Port RE, Ratain MJ, Sheiner LB. A population model for the leukopenic effect of etoposide. Clin Pharmacol Ther. 1995;57(3):325–34.
Beal S, Sheiner L, Boeckmann A, Bauer R. NONMEM User’s Guides (1989–2009). 2009. Icon Dev Solut Ellicott City MD.
Saito S, Kawano Y, Watanabe T, Okamoto Y, Abe T, Kurada Y, et al. Serum granulocyte colony-stimulating factor kinetics in children receiving intense chemotherapy with or without stem cell support. J Hematother. 1999;8(3):291–7.
Takatani H, Soda H, Fukuda M, Watanabe M, Kinoshita A, Nakamura T, et al. Levels of recombinant human granulocyte colony-stimulating factor in serum are inversely correlated with circulating neutrophil counts. Antimicrob Agents Chemother. 1996;40(4):988–91.
Kuwabara T, Kobayashi S, Sugiyama Y. Pharmacokinetics and pharmacodynamics of a recombinant human granulocyte colony-stimulating factor. Drug Metab Rev. 1996;28(4):625–58.
Kuwabara T, Kobayashi S, Sugiyama Y. Kinetic analysis of receptor-mediated endocytosis of G-CSF derivative, nartograstim, in rat bone marrow cells. Am J Physiol. 1996;271(1 Pt 1):E73–84.
Wiczling P, Lowe P, Pigeolet E, Lüdicke F, Balser S, Krzyzanski W. Population pharmacokinetic modelling of filgrastim in healthy adults following intravenous and subcutaneous administrations. Clin Pharmacokinet. 2009;48(12):817–26.
Watari K, Ozawa K, Takahashi S, Tojo A, Tani K, Kamachi S, et al. Pharmacokinetic studies of intravenous glycosylated recombinant human granulocyte colony-stimulating factor in various hematological disorders: inverse correlation between the half-life and bone marrow myeloid cell pool. Int J Hematol. 1997;66(1):57–67.
Fukuda M, Oka M, Ishida Y, Kinoshita H, Terashi K, Fukuda M, et al. Effects of renal function on pharmacokinetics of recombinant human granulocyte colony-stimulating factor in lung cancer patients. Antimicrob Agents Chemother. 2001;45(7):1947–51.
Stute N, Santana VM, Rodman JH, Schell MJ, Ihle JN, Evans WE. Pharmacokinetics of subcutaneous recombinant human granulocyte colony-stimulating factor in children. Blood. 1992;79(11):2849–54.
Extra J-M, Rousseau F, Bruno R, Clavel M, Bail NL, Marty M. Phase I and pharmacokinetic study of taxotere (RP 56976; NSC 628503) given as a short intravenous infusion. Cancer Res. 1993;53(5):1037–42.
Cortes JE, Pazdur R. Docetaxel. J Clin Oncol. 1995;13(10):2643–55.
Burris H, Irvin R, Kuhn J, Kalter S, Smith L, Shaffer D, et al. Phase I clinical trial of taxotere administered as either a 2-hour or 6-hour intravenous infusion. J Clin Oncol. 1993;11(5):950–8.
Tomiak E, Piccart M, Kerger J, Lips S, Awada A, De Valeriola D, et al. Phase I study of docetaxel administered as a 1-hour intravenous infusion on a weekly basis. J Clin Oncol. 1994;12(7):1458–67.
Quartino AL. Pharmacometric models for improved prediction of myelosuppression and treatment response in oncology [internet]. Uppsala: Acta Universitatis Upsaliensis; 2011. Available from: http://urn.kb.se/resolve?urn=urn:nbn:se:uu:diva-150431.
Wallin JE, Friberg LE, Karlsson MO. Model-based neutrophil-guided dose adaptation in chemotherapy: evaluation of predicted outcome with different types and amounts of information. Basic Clin Pharmacol Toxicol. 2010;106(3):234–42.
Wakayama T, Sohmiya M, Furuya H, Murakami Y, Kato Y. Increased serum human granulocyte colony-stimulating factor (G-CSF) levels following intravenous infusion of high-dose methylprednisolone. Endocr J. 1996;43(1):67–72.
Jilma B, Stohlawetz P, Pernerstorfer T, Eichler HG, Müllner C, Kapiotis S. Glucocorticoids dose-dependently increase plasma levels of granulocyte colony stimulating factor in man. J Clin Endocrinol Metab. 1998;83(3):1037–40.
Dexter TM, Simmons P, Purnell RA, Spooncer E, Schofield R. The regulation of hemopoietic cell development by the stromal cell environment and diffusible regulatory molecules. Prog Clin Biol Res. 1984;148:13–33.
Dexter TM. Regulation of hemopoietic cell growth and development: experimental and clinical studies. Leukemia. 1989;3(7):469–74.
ACKNOWLEDGMENTS AND DISCLOSURES
We are grateful to all the patients who kindly participated in the study. We also like to thank Anders Larsson, Åsa Hedlund and Jessica Barrefjord for technical assistance. Model estimation was in part performed on resources provided by Swedish National Infrastructure for Computing (SNIC) through Uppsala Multidisciplinary Center for Advanced Computational Science (UPPMAX). Martin Agback at UPPMAX is acknowledged for assistance concerning technical aspects in making NONMEM run on the UPPMAX resources. The study was supported by the Swedish Cancer Society.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 108 kb)
Rights and permissions
About this article
Cite this article
Quartino, A.L., Karlsson, M.O., Lindman, H. et al. Characterization of Endogenous G-CSF and the Inverse Correlation to Chemotherapy-Induced Neutropenia in Patients with Breast Cancer Using Population Modeling. Pharm Res 31, 3390–3403 (2014). https://doi.org/10.1007/s11095-014-1429-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1429-9