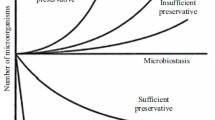
Current data on the use of antimicrobial preservatives in soft dosage forms are presented. Information analysis and analytical studies of regulatory documentation regarding the inclusion of preservatives in the studied group of drugs were carried out. The current range of antimicrobial preservatives included in soft dosage forms is presented. The effectiveness of parabens was evaluated using gel and ointment for topical application as examples.

Similar content being viewed by others
References
State Pharmacopoeia of the Russian Federation, XIVth Ed., Moscow (2018).
Pharmacopoeia of the Eurasian Economic Union (PEAEU), Eurasian Economic Commission, Moscow (2023).
O. M. Khishova, T. V. Bychkovskaya, and A. A. Yaremchuk, Vestn. Farm., 46(4), 97 – 104 (2009).
M. Yu. Pletnev, Preservatives and Current Methods of Protecting Products: Study-Reference Guide [in Russian], Dolgoprudnyi (2013).
O. V. Gunar, G. M. Bulgakova, and N. A. Borisova, Khim.-farm. Zh., 55(4), 56 – 59 (2021).
Good Manufacturing Practice Rules [in Russian], Approved by Order No. 916 (with amendments) of the Ministry of Industry and Trade of the RF, Jun. 14, 2013, Moscow (2013).
Decision No. 77 of the Eurasian Economic Commission Council of Nov. 3, 2016 “On Approval of Good Manufacturing Practice Rules of the Eurasian Economic Union,” Astana (2016).
N. M. Rizaeva, N. A. Yunuskhodzhaeva, R. A. Khusainova, and N. E. Yunuskhozhieva, Farmatsiya, No. 1, 22 – 27 (2023); doi: 10.29296 / 25419218-2023-01-04.
L. V. Kolosova, M. V. Roshchina, and O. V. Gunar, Farmatsiya, 66(7), 8 – 10 (2017).
L. V. Kolosova, Candidate Dissertation in Pharmaceutical Sciences, St. Petersburg (2016).
A. D. Russell, J. Hosp. Infect., 57, 97 – 104 (2004).
PEAEU, Eurasian Economic Commission, Moscow (2020).
Acknowledgments
We thank colleagues and staff of the Microbiology Laboratory,
SCEEMP, Ministry of Health of Russia, for assistance
in performing the experimental studies.
Contributions of authors
LVTs selected and analyzed the preservatives of the studied drugs, was responsible for the accuracy of the results, and wrote the manuscript text; OVG formulated the study plan and reviewed the literature, performed the study determining the effectiveness of the antimicrobial design, edited the manuscript text, and critically reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 57, No. 10, pp. 52 – 56, October, 2023
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tsyganova, L.V., Gunar, O.V. On Antimicrobial Preservatives in Soft Dosage Forms. Pharm Chem J 57, 1661–1665 (2024). https://doi.org/10.1007/s11094-024-03062-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-024-03062-9